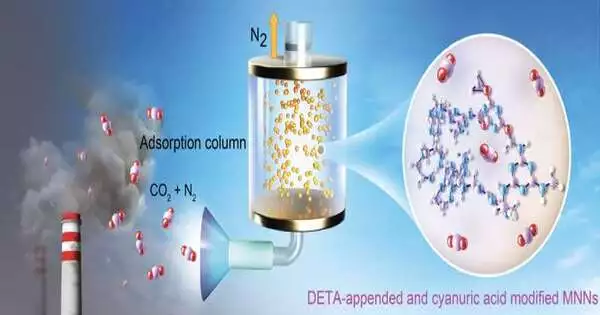
Utilizing a modest polymer called melamine—the primary part of Formica—physicists have made a modest, simple, and energy-effective method for catching carbon dioxide from smokestacks, a vital objective for the United States and different countries as they try to reduce ozone-harming substance outflows.
The cycle for blending the melamine material, described for the current week in the diary Science Advances, might actually be downsized to catch outflows from vehicle exhaust or other mobile wellsprings of carbon dioxide. Carbon dioxide from petroleum products consumed makes up around 75% of all ozone-harming substances created in the U.S.
The new material is easy to make, requiring basically off-the-rack melamine powder — which today costs about $40 per ton — alongside formaldehyde and cyanuric corrosive, a compound that, among different purposes, is added with chlorine to pools.
“We wanted to consider a carbon capture material developed from inexpensive and easily accessible sources. As a result, we chose to begin with melamine.”
Jeffrey Reimer, Professor of the Graduate School in the Department of Chemical
“We needed to ponder a carbon capture material that was gotten from sources that were truly modest and simple to get. Thus, we chose to begin with melamine, “said Jeffrey Reimer, Professor of the Graduate School in the Department of Chemical and Biomolecular Engineering at the University of California, Berkeley, and one of the related creators of the paper.
The alleged melamine permeable organization catches carbon dioxide with a proficiency similar to early outcomes for one more somewhat late material for carbon capture, metal natural systems, or MOFs. UC Berkeley scientists made the main such carbon-catch MOF in 2015, and ensuing forms have been demonstrated to be much more effective at eliminating carbon dioxide from pipe gases, for example, those from a coal-terminated power plant.
However, Haiyan Mao, a UC Berkeley postdoctoral researcher and the paper’s first author, stated that melamine-based materials use significantly less expensive fixings, are simpler to manufacture, and are more energy efficient than most MOFs.The minimal expense of permeable melamine implies that the material could be widely distributed.
“In this review, we zeroed in on a less expensive material plan for catch and capacity and explained the connection system between CO2 and the material,” Mao said. “This work makes an overall industrialization strategy towards a feasible CO2 catch utilizing permeable organizations. We want to believe that we can plan a future connection for catching vehicle fume gas, or perhaps a connection to a structure or even a covering on the outer layer of furniture.
The work is a collaboration among a gathering at UC Berkeley led by Reimer; a gathering at Stanford University driven by Yi Cui, who is head of the Precourt Institute for Energy, the Somorjai Visiting Miller Professor at UC Berkeley, and a previous UC Berkeley postdoctoral individual; UC Berkeley Professor of the Graduate School Alexander Pines; and a gathering at Texas A&M University driven by Hong-Cai Zhou. Jing Tang, a postdoctoral researcher at Stanford and the Stanford Linear Accelerator Center, as well as a meeting researcher at UC Berkeley, is a co-first author with Mao.
Carbon neutrality by 2050
While reducing the consumption of petroleum derivatives is critical for halting environmental change, a significant break system is to capture outflows of carbon dioxide—the super ozone-harming substance—and store the gas underground or convert CO2 into usable items.The U.S. Branch of Energy has previously declared projects adding up to $3.18 billion to help progress and monetarily adaptable advances for carbon catch, use, and sequestration (CCUS) to arrive at an aggressive vent gas CO2 catch proficiency focus of 90%. A definitive U.S. objective is net zero fossil fuel byproducts by 2050.
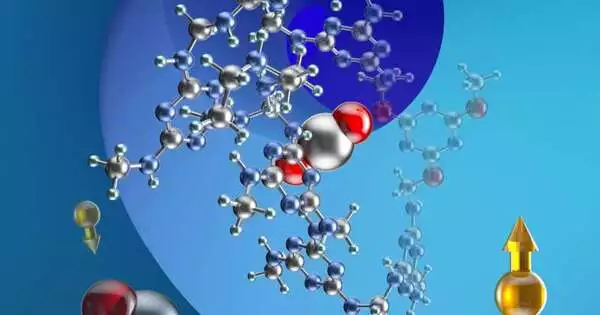
UC Berkeley scientists cultivated a pristine group of feasible, versatile, strong-state materials—polyamine-added, cyanuric corrosive settled, melamine nanoporous networks—that suddenly adsorb CO2 for carbon catch and capacity. In the real world, carbon dioxide atoms (carbon in silver, oxygen in red) connect with amines in the material (nitrogen in blue, hydrogen in green), permitting the material to adsorb the gas from smokestack outflows. The yellow balls with bolts address carbon-13 isotopes and their atomic twists, which were utilized in NMR investigations of the material. Credits: Haiyan Mao and Jeffrey Reimer, UC Berkeley
Yet, carbon capture is nowhere near monetarily feasible. The best method today includes funneling vent gases through fluid amines, which tie CO2. Yet, it requires a lot of energy to deliver the carbon dioxide whenever it’s bound to the amines, so it tends to be focused and put away underground. The amine blend should be warmed to somewhere in the range of 120 to 150 degrees Celsius (250-300 degrees Fahrenheit) to recover the CO2.
Conversely, the melamine permeable organization with DETA and cyanuric corrosive change catches CO2 at around 40 degrees Celsius, somewhat above room temperature, and delivers it at 80 degrees Celsius, below the limit of water. The energy reserve funds come from not warming the substance to high temperatures.
In its exploration, the Berkeley/Stanford/Texas group zeroed in on the normal polymer melamine, which is utilized in Formica as well as cheap dinnerware and utensils, modern coatings, and different plastics. Treating melamine powder with formaldehyde — which the analysts did in kilogram amounts — makes nanoscale pores in the melamine that the scientists thought would retain CO2.
Mao said that tests affirmed that formaldehyde-treated melamine adsorbed CO2 fairly, yet adsorption could be significantly better by adding another amine-containing compound, DETA (diethylenetriamine), to tie up carbon. She and her colleagues discovered that adding cyanuric corrosive during the polymerization response significantly increased pore size and profoundly improved CO2 catch proficiency: nearly all of the carbon dioxide in a mimicked vent gas blend was consumed in around 3 minutes.
The expansion of cyanuric corrosion likewise permitted the material to be utilized again and again.
Mao and her colleagues conducted strong state atomic attractive reverberation (NMR) studies to understand how cyanuric acid and DETA are linked to make carbon capture so effective.The examinations showed that cyanuric corrosive structures form solid hydrogen bonds with the melamine network that settles DETA, keeping it from draining out of the melamine pores during rehashed patterns of carbon catch and recovery.
“What Haiyan and her partners had the option to show with these rich methods is precisely the way in which these gatherings mix, precisely the way that CO2 responds with them, and that within the sight of this pore-opening cyanuric corrosive, she’s ready to cycle CO2 on and off, commonly with a limit that is actually very great,” Reimer said. Also, the rate at which CO2 adsorbs is very fast, compared with a few different materials. Thus, every one of the useful angles at the lab size of this material for CO2 capture has been met, and it’s simply amazingly modest and simple to make. “
“We efficiently clarified the system of the response of the nebulous organizations with CO2 using strong state atomic attractive reverberation methods,” Mao said.”For the energy and natural local area, this work makes an elite exhibition, strong state network family along with a careful comprehension of the systems, and in addition, empowers the development of permeable materials research from experimentation strategies to sane, bit by bit, nuclear level tweak.”
The Reimer and Cui bunches are proceeding to change the pore size and amine concentrations to further develop the carbon capture proficiency of melamine permeable organizations while keeping up with energy productivity. This includes utilizing a method called dynamic combinatorial science to shift the extent of fixings to accomplish a viable, versatile, recyclable and high-limit CO2 catch.
Reimer and Mao have likewise firmly teamed up with the Cui group at Stanford to blend different kinds of materials, including progressive nanoporous films—a class of nanocomposites joined with a carbon circle and graphene oxide—and various leveled nanoporous carbons produced using pine wood, to adsorb carbon dioxide. Reimer created strong state NMR explicitly to portray the system in which strong materials connect with carbon dioxide to better configuration materials for carbon capture from the climate and energy stockpiling. Cui fostered a strong and feasible strong state stage and manufacturing methods for establishing new materials to address environmental change and energy stockpiling.
More information: Haiyan Mao et al, A scalable solid-state nanoporous network with atomic-level interaction design for carbon dioxide capture, Science Advances (2022). DOI: 10.1126/sciadv.abo6849
Journal information: Science Advances