Over the course of the last many years, architects and materials researchers have been endeavoring to foster battery innovations that show progressively better exhibitions. Their endeavors are directed toward supporting the necessities of the gadget business, expanding the battery duration of innumerable battery-powered gadgets, electric vehicles, and mechanical frameworks.
Nickel (Ni)-rich layered materials have been frequently utilized as cathodes in battery cells because of their profitable properties that can build the cells’ energy thickness. In any case, the limit of Ni-rich cathodes can quickly decay because of the materials’ reactivity, which causes unwanted side-substance responses.
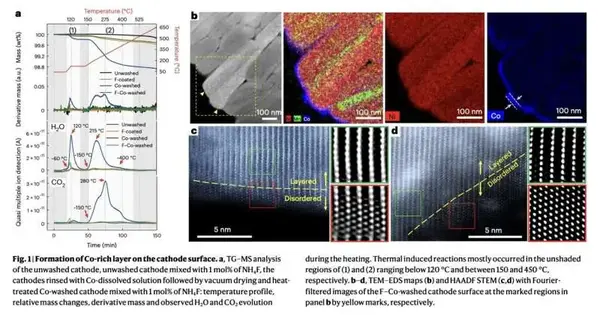
Specialists at Hanyang College in Seoul as of late presented another system that could assist with improving the presentation of these cathodes, permitting them to hold their ability for longer timeframes. This methodology, presented in a paper distributed in Nature Energy, includes a basic washing process that assists with eliminating lingering lithium intensities on the cathodes while likewise covering them to diminish outlandish responses.
“The instability of Ni-rich layered cathode materials in lithium-ion batteries is attributed to their labile surface reactivity. This reactivity induces the formation of residual lithium impurities on the cathode surface as well as severe side reactions with the electrolyte. We suggest a washing procedure that uses Co-dissolved water to remove leftover lithium while also producing a protective covering on Ni-rich multilayer cathodes.”
Hoon-Hee Ryu, Hyung-Woo Lim and their colleagues wrote in their paper.
“The precariousness of the Ni-rich layered cathode materials in lithium-particle batteries is credited to their labile surface reactivity.” Hoon-Hee Ryu, Hyung-Charm Lim, and their partners wrote in their paper. “This reactivity prompts the development of remaining lithium pollutants on the cathode surface and serious side reactions with the electrolyte. We propose a washing interaction involving co-broken-up water while at the same time eliminating leftover lithium and shaping a defensive covering on Ni-rich layered cathodes.”
The critical target of the new work by this group of scientists was to recognize a powerful technique to beat the limits of Ni-rich cathodes, which could thusly assist with expanding the term of batteries in time. Their proposed methodology involves washing the cathodes with a cobalt (Co)-broken-up watery arrangement.
This washing system evokes a response with the remaining lithium on the external surface of the cathodes, prompting the development of a dainty, uniform, and CO-rich defensive layer. The framed layer disposes of direct contact between the cathode and the electrolyte inside the battery cell, accordingly forestalling further side responses and the subsequent fast debasement of the cathode over the long run.
“The washing incites the remaking of the close surface design through responses with the remaining lithium compounds, subsequently forestalling direct contact between the electrolyte and the Ni-rich surface,” Ryu, Lim, and their associates made sense of. “An extra fluorine covering on the washed cathode blocks the decay of salts, forestalling the results from setting off autocatalytic side responses at the electrolyte-cathode interface and subsequently stifling gas age during cycling.”
The washing and covering methodology presented by Ryu, Lim, and their colleagues was tried in a progression of starting tests. Results showed that it effectively broadened the existence pattern of Ni-rich cathodes without undermining their energy thickness or wellbeing.
Later on, this new work could be utilized to foster more secure and solid batteries for electric vehicles and other enormous-scope hardware. Likewise, other exploration groups could draw motivation from its discoveries to devise other promising techniques to forestall the fast limit weakening usually detailed in Ni-rich cathodes.
More information: Hoon-Hee Ryu et al, Near-surface reconstruction in Ni-rich layered cathodes for high-performance lithium-ion batteries, Nature Energy (2023). DOI: 10.1038/s41560-023-01403-8.