As opposed to being exclusively negative, breaks in the positive cathode of lithium-particle batteries lessen battery charge time, research done at the College of Michigan shows.
This is in opposition to the viewpoint of many manufacturers of electric vehicles, who strive to reduce cracking because it shortens the lifespan of batteries.
“Many organizations are keen on making ‘million-mile’ batteries utilizing particles that don’t break. The study’s corresponding author, assistant professor of materials science and engineering Yiyang Li, stated, “Unfortunately, if the cracks are removed, the battery particles will not be able to charge quickly without the additional surface area from those cracks.” We don’t want to have to wait five hours for a car to charge on a road trip. Within fifteen to thirty minutes, we want to charge.”
“Many companies are interested in developing’million-mile’ batteries with non-cracking particles. Unfortunately, without the extra surface area provided by the fissures, the battery particles will be unable to charge quickly.”
Yiyang Li, assistant professor of materials science and engineering
The group accepts that the discoveries apply to the greater part of all electric vehicle batteries, where the positive terminal—or cathode—is made out of trillions of infinitesimal particles made of either lithium nickel manganese cobalt oxide or lithium nickel cobalt aluminum oxide.
Hypothetically, the speed at which the cathode runs depends on the particles’ surface-to-volume proportion. Because of their greater surface area-to-volume ratio, smaller particles should be able to charge more quickly than larger ones, allowing lithium ions to diffuse over shorter distances.
However, conventional methods could only average the charging properties of the battery’s cathode and could not directly measure the charging properties of individual cathode particles. Because of this restriction, the well-known connection between charging speed and cathode particle size was only an assumption.
“We observe that the cathode particles are broken and have more dynamic surfaces to take in lithium particles—on their external surface, yet inside the molecule breaks,” said Jinhong Min, a doctoral understudy in materials science and design working in Li’s lab. “Battery researchers realize that breaking happens, yet they have not estimated how such breaking influences the charging speed.”
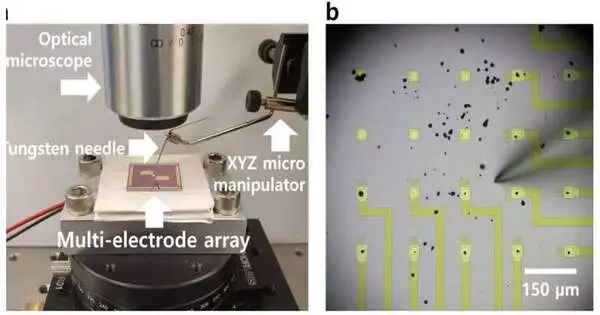
Estimating the charging rate of individual cathode particles was vital to finding the potential gain from breaking cathodes, which Li and Min achieved by embedding the particles into a gadget that is normally utilized by neuroscientists to concentrate on how individual synapses send electrical signals.
“A while ago, when I was in graduate school, a partner concentrating on neuroscience showed me these clusters that they used to concentrate on individual neurons. Li said, “I was wondering if we could also use them to study battery particles, which are about the same size as neurons.”
A two-by-two-centimeter chip with up to one hundred microelectrodes makes up each array, which was designed specifically for it. In the wake of dissipating some cathode particles in the focal point of the chip, Min moved single particles onto their own terminals on the exhibit using a needle multiple times more slender than a human hair. Min measured 21 particles in this particular study and was able to simultaneously charge and discharge up to four individual particles on the array once the particles were in place.
The investigation uncovered that the cathode particles’ charging speeds didn’t depend on their size. According to Li and Min, the most likely cause of this strange behavior is that when larger particles crack, they behave like a collection of smaller particles. Another chance is that the lithium particles move rapidly in the grain limits—the little spaces between the nanoscale precious stones containing the cathode molecule.
Li believes that this is unlikely unless cracks are formed by the battery’s electrolyte, the liquid medium in which the lithium ions move.
The advantages of broken materials are vital to consider while planning seemingly perpetual batteries with single-gem particles that don’t break. To charge rapidly, these particles might be more modest than the present breaking cathode particles. The option is to make single-gem cathodes with various materials that can move lithium quicker; however, those materials could be restricted by the inventory of essential metals or have lower energy densities, Li said.
More information: Jinhong Min et al, Direct measurements of size-independent lithium diffusion and reaction times in individual polycrystalline battery particles, Energy & Environmental Science (2023). DOI: 10.1039/D3EE00953J