Torment has been for some time perceived as one of advancement’s most solid devices to identify the presence of damage and a sign that something is off-base — a ready framework that advises us to delay and focus on our bodies.
Yet, imagine a scenario where torment is something beyond a simple alert. Imagine a scenario where torment is in itself a type of security.
Another study led by researchers at Harvard Clinical School suggests that this is most likely the case in mice.The study, published Oct. 14 in Cell, shows that aggravation neurons in the mouse stomach direct the presence of defensive bodily fluid under typical circumstances and animate digestive cells to deliver more bodily fluid during conditions of irritation.
The work subtleties the means of a perplexing flagging fountain, showing that aggravation neurons take part in direct crosstalk with bodily fluid-containing stomach cells, known as cup cells. It just so happens, agony might safeguard us in more straightforward ways than its exemplary task of identifying likely damage and dispatching signs to the mind. “Our work shows how tormented interceding nerves in the stomach converse with adjacent epithelial cells that line the digestion tracts,” said concentrate on senior agent Isaac Chiu, academic partner of immunology in the Blavatnik Foundation at HMS. “This implies that the sensory system plays a significant part in the stomach’s past, giving us a terrible sensation and that it’s a central member in stomach boundary upkeep and a defensive component during irritation.”
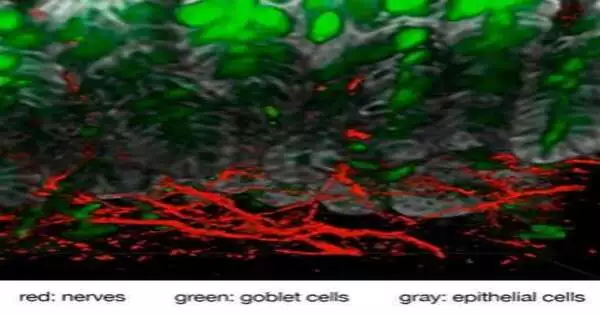
Harvard Clinical School analysts have examined the atomic crosstalk between torment strands in the stomach and the cup cells that line the walls of the digestive tract. The work shows that compound signs from tormented neurons incite cup cells to deliver defensive bodily fluid that covers the stomach and safeguards it from harm. The discoveries show that digestive aggravation isn’t a simple location and-flagging framework, yet assumes a direct defensive part in the stomach. Chiu Laboratory/Harvard Clinical School
An immediate discussion
Our digestion tracts and aviation routes are studded with cup cells. Named for their cup-like appearance, cup cells contain gel-like bodily fluid made of proteins and sugars that acts as a defensive covering that safeguards the outer layer of organs from scrapes and harm. The new exploration found that digestive cup cells discharge defensive bodily fluid when set off by direct connection with torment-detecting neurons in the stomach.
“This finding indicates that these nerves are activated not only during acute inflammation, but also at rest. The presence of normal gut microbes appears to tickle the nerves and cause the goblet cells to release mucus.”
senior investigator Isaac Chiu, associate professor of immunobiology in the Blavatnik Institute at HMS.
In a bunch of tests, the scientists saw that mice lacking torment neurons created less defensive bodily fluid and experienced changes in their digestive microbial communities—an unevenness in useful and unsafe organisms known as dysbiosis. To explain exactly how this defensive crosstalk happens, the analysts examined the behavior of cup cells in the presence of torment neurons.
They found that the surfaces of cup cells contain a kind of receptor, called RAMP1, that guarantees the phones can answer nearby tormented neurons, which are enacted by dietary and microbial signs, as well as mechanical strain, compound bothering, or radical changes in temperature. The tests additionally showed that these receptors interface with a compound called CGRP, delivered by neighboring tormented neurons when the neurons are invigorated. The RAMP1 receptors, the analysts found, are likewise present in both human and mouse cup cells, hence making them receptive to torment signals.
Tests additionally showed that the presence of specific stomach organisms enacted the arrival of CGRP to keep up with stomach homeostasis.
“This finding lets us know that these nerves are set off by intense irritation, yet in addition to that,” Chiu said. “Simply having normal stomach organisms around seems to stimulate the nerves and makes the cup cells discharge bodily fluid.” Chiu said that this input circle guarantees that microorganisms sign to neurons, neurons direct the bodily fluid, and the bodily fluid keeps stomach organisms solid.
The review revealed that, in addition to microbial presence, dietary factors played a role in activating torment receptors.When analysts gave mice capsaicin, the primary fixing in stew peppers known for its capacity to set off extreme, intense agony, the mice’s aggravation neurons got quickly enacted, making cup cells discharge bountiful measures of defensive bodily fluid.
Conversely, mice lacking either torment neurons or cup cell receptors for CGRP were more helpless to colitis, a type of stomach irritation. The finding could make sense of why individuals with stomach dysbiosis might be more inclined to colitis. When analysts gave torment-flagging CGRP to creatures lacking agony neurons, the mice experienced fast improvement in bodily fluid creation. The treatment safeguarded mice against colitis even without tormenting neurons.
The finding shows that CGRP is a vital provocateur of the flagging fountain that prompts the emission of defensive bodily fluid.
“Torment is a typical side effect of ongoing fiery states of the stomach, like colitis, yet our review shows that intense aggravation assumes a direct defensive role too,” said concentrate on first creator Daping Yang, a postdoctoral scientist in the Chiu Lab.
A potential drawback to stifling agony
The group’s tests showed that mice lacking agony receptors likewise had more awful harm from colitis when it happened. Considering that aggravation meds are many times used to treat patients with colitis, it could be vital to consider the conceivable adverse effects of impeding agony, the analysts said.
“In individuals with irritation of the stomach, one of the significant side effects is torment, so you could feel that we’d need to treat and impede the aggravation to ease the torment,” Chiu said. Yet, some pieces of this aggravation sign could be straightforwardly defensive as a brain reflex, which brings up significant issues about how to painstakingly oversee torment in a manner that doesn’t prompt different damage.”
Moreover, a class of normal headache meds that stifle the emission of CGRP might harm stomach boundary tissues by impeding this defensive torment flagging, the scientists said.
“Considering that CGRP is a go-between of cup cell capability and bodily fluid creation, assuming we are constantly impeding this defensive system in individuals with headaches and assuming they are taking these meds long haul, what occurs?” Chiu said. “Are the medications going to impede the mucosal coating and individuals’ microbiomes?”
Cup cells have various different capabilities in the stomach. They give entry to antigens—proteins found on infections and microbes that start a defensive safe reaction by the body—and they produce antimicrobial synthetics that shield the stomach from microorganisms. “One inquiry that emerges from our ongoing work is whether torment strands likewise manage these different elements of cup cells,” Yang said.
Yang added that a different line of request is to investigate disturbances in the CGRP flagging pathway and decide if glitches are impacting everything in patients with hereditary inclination to fiery gut illness.
More information: Isaac M. Chiu, Nociceptor neurons direct goblet cells via a CGRP-RAMP1 axis to drive mucus production and gut barrier protection, Cell (2022). DOI: 10.1016/j.cell.2022.09.024. www.cell.com/cell/fulltext/S0092-8674(22)01196-5
Journal information: Cell