Astrocytes, a type of glial cell found in the central nervous system, are responsible for clearing out excess neurotransmitters, promoting the formation of synapses—the connections between neurons—and carrying out various other tasks. The improvement of astrocytes, similar to that of other glial cells, is incompletely upheld by extraneous ligands. Ligands are particles that tight spot to a getting protein, known as a receptor, getting different cell reactions.
Scientists at Emory College Institute of Medication as of late decided to distinguish ligand-receptor coordinates that help the beginning and improvement of astrocytes in the human mind utilizing computational methods. Their paper, distributed in Nature Neuroscience, features the capability of examining a lot of accessible information to distinguish new neuroscientific speculations.
One of the researchers who carried out the study, Steven A. Sloan, told Medical Xpress, “This work was initially inspired by our need to pivot our lab efforts with the onset of the COVID pandemic.” We have a longstanding interest in how the destinies of neurons and astrocytes are chosen during early mental health. It’s exceptional, on the grounds that these phone types share similar begetters, however those forebears totally change their destiny from making neurons to astrocytes during a particular formative time window.”
“We’ve long been interested in how neurons and astrocytes decide their fates during early brain development. It’s fascinating because different cell types share the same progenitors, yet those progenitors entirely switch their fate from generating neurons to making astrocytes during a precise developmental time frame.”
Steven A. Sloan, one of the researchers who carried out the study,
The “gliogenic switch” through which begetter cells at last produce astrocytes has been examined for a really long time. Previous research in this area has revealed a number of molecular changes and secreted molecules that may support this switch, which ultimately alters progenitor cell fate.
Sloan explained, “We figured there must be more molecular changes that haven’t been identified yet.” Since then, there has been an explosion of genomic data, particularly single cell sequencing of the fetal brain. The majority of these studies took place over a decade ago. As a result, when we were unable to conduct experiments in the lab during COVID, we believed we could mine these datasets to determine which molecules might be communicating with one another in the developing brain to shift progenitor fates away from neurogenesis and toward astrogenesis.”
The new work by Sloan and his partners was motivated by a past paper distributed in Nature Strategies, which acquainted a calculation with break down organic and genomics information. NicheNet, a complicated algorithm, had been used to address other research questions unrelated to astrocyte development up until this point.
“NicheNet is a muddled calculation that has a moderately straightforward clarification,” Sloan said. ” Computationally, we previously needed to enter RNA-seq (single cell) information of cells that we guessed may be conveying a message. For our situation, these were the cells in the mind that are available before astrogenesis (for the most part, youthful neurons). Then, we needed to characterize the phones that may be getting the signs (ancestors called spiral glia).”
After the analysts took care of the calculation important RNA-seq information and characterized cells that could be getting signals from them, NicheNet investigated the information. Basically, it attempted to coordinate atoms with receptors, recognizing particles could have been discharged by source cells that tight spot to receptors communicated on collector cells. A list of possible ligand-receptor pairs involved in the genesis of astrocytes was compiled through this analysis.
“We then made this one stride further: Sloan explained, “We could filter them by their predicted ability to turn on astrocyte genes, rather than just picking some of these candidates.” This aided a ton, significantly reducing our selected rundown of up-and-comer particles. There were some in that rundown we perceived from the writing, and others that were totally new to us. We figured we could attempt to find a little mixed drink of ligands that could act correlative to one another, with the possibility that these particles likely don’t act in separation.”
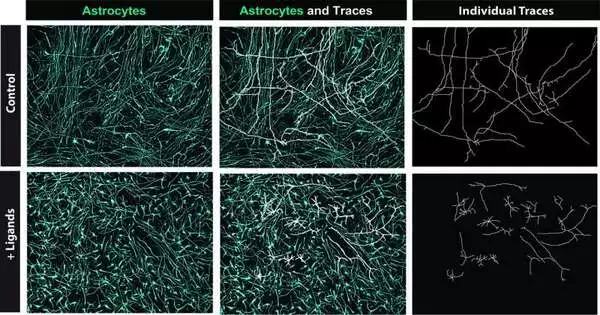
The researchers discovered five ligands from the NicheNet algorithm’s output, each of which would activate a different set of astrocyte gene expression. Notably, there would be no overlap in the genes that were activated by these ligands.
Sloan and his partners added these five ligands to organoids (i.e., worked on variants of organs made in lab settings) that they were filling in their lab. They used RNA-sequencing on the organoids after 30 days to see if the ligands had a positive effect on progenitor cell fate and accelerated astrocyte formation.
“We noticed a significant upregulation of astrocyte qualities and downregulation of neuronal qualities,” Sloan said. ” It truly appeared to be that the ligands were moving cell destiny! We repeated this experiment multiple times to determine when the ligands have the greatest effect. It appears that this occurs as the expression of the receptors gets closer to the gliogenic switch. In addition, we determined that the cocktail was more effective in eliciting this astrocyte commitment when compared to the sequential addition of each of the five ligands.
The specialists did a few extra trials to approve the impacts they saw in the organoids. In particular, they presented the five ligands they recognized in human cells and saw what occurred.
“At long last, we attempted to limit which sub-atomic pathways these ligands were actuating to push forebears towards an astrocyte destiny,” Sloan said. ” We questioned a grouping of normal flagging pathways with a little screen and tracked down bunches of changes. However, a pathway known as mTORC1, which had previously been linked to astrocyte biology, stood out to us. Subsequently, we further approved enactment of this pathway upon ligand excitement and designate it as a putative controller of astrocyte improvement in people.”
By breaking down existing datasets utilizing the NicheNet calculation, Sloan and his partners had the option to outline a speculation that they then, at that point, tried in their lab. As a result, their paper reaffirms the enormous potential of computational methods for neuroscience research.
“Research bunches are proceeding to spill out an ever increasing number of huge information with the expectations that they’ll prompt new testable speculations and investigations,” Sloan said. ” Here, we’re really keeping that word. Our review distinguishes novel gliogenic ligands, yet absolutely its greater picture application is the way that others could utilize these equivalent datasets to pose many different inquiries about how cell correspondence shapes early mental health.”
Future examinations could investigate the conceivable job of the ligand-receptor matches distinguished by this group of specialists in the further advancement of astrocytes. In addition, the findings of this most recent study may serve as a model for applying NicheNet or other algorithms of a similar nature to other pressing issues in neuroscience, such as neuroimmunology, myelination, neuronal specification, and the diseased brain.
Sloan continued, “Our most recent study primarily focused on the extrinsic cues that influence astrocyte development.” We are eager to learn more about the intrinsic cellular states and signals that enable a radial glia progenitor to switch from neurogenic to gliogenic states in the future. Since this switch appears to be involved in and disrupted in nearly all neurodevelopmental disorders, it is crucial to comprehend the mechanisms governing this fate decision.”
More information: Anna J. Voss et al, Identification of ligand–receptor pairs that drive human astrocyte development, Nature Neuroscience (2023). DOI: 10.1038/s41593-023-01375-8
Five molecules work together to drive human astrocyte development, Nature Neuroscience (2023). DOI: 10.1038/s41593-023-01391-8