Is it conceivable that, in many estimations in the field of life sciences, significant connections stay concealed inside the cell or at the cell surface? This question has baffled the group of laser and biophysicists led by Prof. Dr. Alexander Rohrbach from the College of Freiburg for a really long time. Together with his colleague Dr. Felix Jünger, they have been looking into a variety of interactions that occur on various cell surfaces between particles that are within the size range of bacteria (a few micrometers) and even viruses (0.1 micrometers).
They have now been successful in making interactions that were previously invisible visible through the application of sophisticated laser measurement technology and mathematical analysis techniques. The outcomes have been distributed in the diary. They might aid in gaining a better understanding of how various particles, such as micelle-coated active ingredients, viruses, bacteria, fine dust, cell debris, or others, bind to cells in the future.
“During signal transduction, the extracellular matrix distinguishes particles not only by their size, shape, and surface, but also by how quickly or slowly thermally diffusing particles contact the surface, i.e., it acts as a spatial and temporal sieve for mechanical signals,”
Bio-physicist Prof. Dr. Alexander Rohrbach from the University of Freiburg
Viscoelasticity decides conduct.
The viscoelastic properties of cell surfaces assume an unequivocal role here. A starch solution is used as an illustration by Rohrbach. You can actually walk on water if enough corn starch is added to a tub of water and you quickly walk on it. All you feel is a flexible surface. However, if you slow down or stop walking, you sink in and feel the liquid around your feet. Therefore, the solution can be either elastic or viscous, depending on the acting time scale. The watcher sees either an individual with dry or wet feet getting out of the tub.
Organic cells comprise momentary atomic designs, which are just noticeable under the best magnifying instruments. They all respond to tension or pressure in two ways: viscously (energy is lost) and elastically (energy is stored). Every individual cell is a viscoelastic framework, which consequently determines the viscoelastic properties, for instance, of muscle or connective tissue.
The extracellular matrix of nearly every cell is extremely specialized: a web of molecules that look like filaments, fine fibers, and protrusions that look like tiny fingers. Particles like viruses, bacteria, and particulates are attracted by this complex cell surface. During signal transduction, the extracellular network recognizes not just the size, shape, and surface of the particles, but also how rapidly or gradually thermally diffusing particles contact the surface, i.e., it goes about as a spatial and transient sifter for mechanical signs,” Rohrbach makes sense of.
Incredible quick estimation innovation
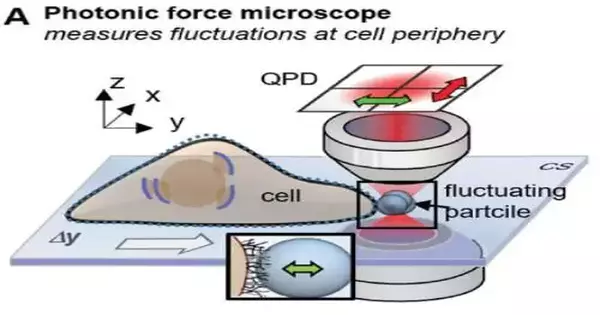
Utilizing a purported photonic force magnifying lens, a blend of optical laser tweezers, and an interferometric 4-quadrant location framework, bio-physicist Jünger originally moved toward one-micrometer dots in living cells in various trials. The molecule, which fills in as a test, is caught in the intense attention yet at the same time makes little shuddering developments, known as warm position changes.
With a precision of a few nanometers, these movements are measured in three dimensions one million times per second. The loud-looking position signs of the molecule, notwithstanding, contain significant data about the connection with its current circumstance.
On the off chance that the molecule is currently leisurely carried to the cell surface with optical tweezers, or, on the other hand, assuming the molecule is introduced to the cell at a short, consistent distance, a collaboration of the molecule with the fine designs of the extracellular network starts following a couple of moments. On the off chance that you take a gander at the position histogram, which is a circulation of all molecule positions, you will see essentially no distinction in the dissemination when the connection The conversation goes unnoticed.
“The quick sign inspection permitted us to perform Kramers-Kronig fundamental changes for warm molecule vacillations interestingly, which permit us to picture the flexible and gooey way of behaving of cell structures at various frequencies of movement,” Jünger makes sense of.
Rohrbach adds, “Be that as it may, to give significance to these two recurrence reactions, you need to pack your concept of what’s going on at the atomic level into numerical conditions and afterward contrast how well the arrangements with these situations match the exploratory outcomes.”
Numerical negligible model in the recurrence area
The Freiburg specialists have hence conceived a numerically negligible model in recurrence space that can be out of the blue very much adjusted to the viscoelastic conduct in various estimation techniques on various cells. By breaking down the test changes, Jünger and Rohrbach had the option to decide, for instance, on the significant properties of the pericellular grid (PCM) in gastrointestinal epithelial cells, which comprises lattice-like hyaluronic corrosive strands.
“We had the option to quantify the thickness of the PCM at 350 nanometers on the microsecond scale alone; on longer time scales, the PCM was just undetectable,” Rohrbach says. Small, highly dynamic viruses tend to bounce off the PCM more elastically from a physical standpoint, whereas larger, less dynamic bacteria tend to sink into the PCM precisely because it is less elastic on larger time scales. This could be explained by the PCM’s elasticity of only about six pascals at millisecond dynamics and about 20 pascals at microsecond dynamics.
More information: Felix Jünger et al, Making Hidden Cell Particle Interactions Visible by Thermal Noise Frequency Decomposition, Small (2023). DOI: 10.1002/smll.202207032