By concentrating on uncommon instances of indistinguishable twins with leukemia, researchers have revealed new insight into the beginnings of the most well-known kind of young-life disease, confirming that it starts in the belly and that circumstances after birth determine if clinical leukemia develops.
Discoveries from analysts at The Foundation for Disease Exploration, London, will assist clinicians with informing guardians regarding twin kids with intense lymphoblastic leukemia (ALL), offering direction on risk level and screening.
The first-of-its-kind study, which was distributed in the diary Leukemia, involved seven sets of twins with “harsh” ALL, implying that at first only one twin in each pair had the illness.
The scientists followed the twins for as long as 15 years to comprehend the organic motivation behind why a generally sound twin is at a higher risk of fostering ALL later in the event that their indistinguishable twin has previously been determined to have the illness.
“The most prevalent kind of cancer in kids is acute lymphoblastic leukemia (ALL), which strikes kids more frequently than adults. It’s crucial to comprehend the mechanism underlying how cancer manifests in identical twins and why frequently only one develops leukemia. It gives us insight into how leukemia develops in all children as well as the likelihood that the other sibling will also have the disease.”
Sarah McDonald, deputy director of research at Blood Cancer UK
That possibility is estimated to be 15-25%, as opposed to less than 1% for a non-distinguishable twin or other kin.However, as this study confirms, that high risk may apply if the indistinguishable twins shared a single placenta prior to birth.
ALL is expected to be triggered by two “hits” of hereditary changes.
A previous study by a similar group proposed that two “hits” of hereditary changes should “trigger” ALL.The new findings confirm that the main hereditary change occurs in the stomach and is shared by platelets from the two twins via their normal blood supply.The subsequent change, assuming it works out, happens after birth.
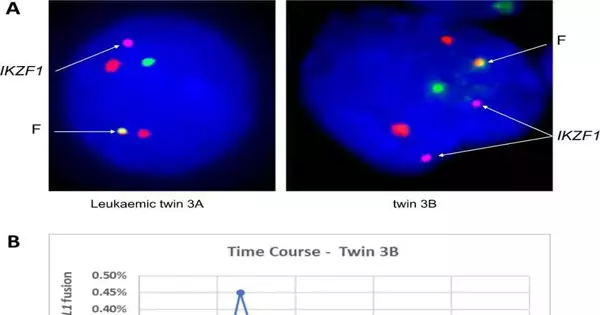
The new examination shows that the solid twin of a patient with ALL has low yet noticeable degrees of “clinically quiet” yet freak “pre-leukemia” cells in the blood. These cells convey precisely the same “first hit” change as the co-twin with ALL, and that implies they can be estimated and checked. In the event that those calls increase in number, this might flag the improvement of ALL, provoking a bone marrow biopsy and possibly early treatment.
However, assuming the sound twin has consistent low levels of such cells that don’t increment or even decay, this provides clinicians and guardians with reassurance that everything isn’t creating.
Until now, two sound co-twins engaged in this review—wwho had low levels yet noticeable degrees of pre-leukemia cells in their blood—hhad later been diagnosed with ALL.
Affirmation that all life begins in the belly
That is what the discovery confirms—much like instances of young life ALL in indistinguishable twin matches or singletons, these instances of “harsh” ALL in twins begin in the belly.
The fact that occasionally only one indistinguishable twin fosters Everything is thus due to the requirement (for one twin) of the critical second post-birth “hit,” or change, which is thought to be set off — most likely by normal adolescent diseases, as per other research from the gathering that drove this review.
Other research by the group has revealed that a large number of children are born with the first mutational “hit,” yet only a small percentage (around 1%) go on to have ALL.Research by the group is presently centered around understanding what determines the probability of this subsequent disease-driven “hit” during the early, formative years.
Surveying risk more precisely
The discoveries have suggestions for clinicians and guardians of twin kids with ALL. Previously, guardians were given varying advice on the risk to any solid twin when the other fosters the illness.
The new findings suggest that the gamble can be more precisely assessed—by first determining whether the twins are indistinguishable and shared a placenta, and then sequentially looking at them—by tracking the levels of pre-leukemia cells in their blood.
At the point when one of the twins remains clinically well, analysts suggest re-screening like clockwork—tto monitor the degree of pre-leukemia cells and whether they are expanding. Hazard to the subsequent twin is most prominent in the main year after the primary twin is analyzed and declines after that, implying that screening of asymptomatic, sound twins is likely pointless after the age of ten.
New insights into the beginnings of ALL
Teacher Sir Mel Greaves, Establishing Head of the Middle for Advancement and Disease and Teacher of Cell Science at the Foundation of Malignant Growth Exploration, London, said,
“Our review gives new experiences into the beginnings of young-onset intense lymphoblastic leukemia, the most well-known kind of young-onset disease, representing 80% of leukemia cases in kids.
“Our past discoveries showed that leukemia in twins is due to the sharing of platelets inside a solitary placenta before birth, as opposed to hereditary inheritance.” These new discoveries affirm that the illness can be followed back to the belly when pre-leukemia cells spread through the twins’ common blood supply.
“What remained a mystery until recently was the reason that occasionally only one twin is determined to have leukemia, leaving clinicians in the dark when it comes to offering counsel and consolation to guardians.”In this new review, we give a natural clarification to what occurs in this situation and show that the solid twin frequently has a few pre-leukemia cells in their blood, which have similar hereditary markers as the disease cells in the wiped-out twin.
“By estimating the levels of pre-leukemia cells in the blood, we can precisely survey the risk of the illness and guide screening and checking.”If one twin develops ALL, the other twin is more likely to develop leukemia—even if the twins shared a placenta in the belly, which only happens in about 60% of identical twin matches.
“We actually don’t know for specific what prompts the first “hit” of hereditary changes in the belly, yet we feel that the second “hit” of hereditary changes is likely set off by normal youth contaminations, opening up the chance of “preparing” a framework at the outset to stay away from the improvement of the illness further down the road.”Our flow research proposes that the stomach microbiome might be playing a vital role in this cycle.”
Assisting clinicians with giving direction
Sarah McDonald, agent head of exploration at Blood Disease UK, said, “Intense lymphoblastic leukemia (everything) is the most well-known sort of malignant growth in kids, influencing youngsters more frequently than grown-ups.” Understanding how the disease causes indistinguishable twins and why only one frequently develops leukemia is a critical question to address.It helps us understand both the risk of other relatives developing leukemia and how leukemia develops in all children.
“This examination shows that in situations where one twin creates leukemia and the two twins both offer a placenta during pregnancy, two occasions are expected to determine if the other kin fosters the illness—oone preceding birth and the other later.” This work, made possible by research volunteers with leukemia over many years, allows for the possibility of determining whether the unaffected kin has pre-leukemia cells in their blood.Assuming they do, it might imply that they can be firmly checked and treated early, assuming it seemed as though they planned to foster leukemia, allowing them the most ideal opportunity for endurance. This discovery could also help clinicians advise families on the likelihood of their children having children.
“For Blood Disease UK, we need to thank our allies, who empower us to spearhead research like this. While this investigation adds to our understanding of how youth everything starts, there is still work to be done to complete the picture—to get to the point where no lives are lost due to blood disease.
More information: Anthony M. Ford et al, Covert pre-leukaemic clones in healthy co-twins of patients with childhood acute lymphoblastic leukaemia, Leukemia (2022). DOI: 10.1038/s41375-022-01756-1
Journal information: Leukemia