In ACS Nano, researchers from Kanazawa University explain how the chemical reduction of carbon dioxide can be sped up by using ultrathin layers of tin disulfide. This is an important finding for our quest for a carbon-neutral society.
In humanity’s urgent quest for a carbon-neutral, sustainable society, recycling carbon dioxide (CO2) released by industrial processes is a necessity. Electrocatalysts that are able to convert CO2 into other chemical products with lower environmental impacts are currently the subject of extensive research for this purpose. Two-dimensional (2D) metal dichalcogenides, a class of materials, are potential electrocatalysts for CO2 conversion. However, these materials typically also facilitate competing reactions, which reduces their efficiency.
A 2D metal dichalcogenide has been discovered by Yasufumi Takahashi and colleagues from Kanazawa University’s Nano Life Science Institute (WPI-NanoLSI) that can effectively convert CO2 into formic acid, a compound that not only occurs naturally but also serves as an intermediate product in chemical synthesis.
The catalytic performance of tin disulfide (SnS2) and 2D sheets of disulfide (MoS2) was compared by Takahashi and colleagues. Both are 2D metal dichalcogenides, with the last option exceptionally compelling in light of the fact that unadulterated tin is a known impetus for the creation of formic corrosives. These compounds were subjected to electrochemical testing, which demonstrated that MoS2 promoted the hydrogen evolution reaction (HER) rather than CO2 conversion. The term “HER” refers to a reaction that produces hydrogen. This reaction can be useful for the purpose of producing hydrogen gas fuel; however, in the context of reducing CO2, it is an undesirable competing process. SnS2, on the other hand, suppressed HER and had good CO2 reduction activity. The bulk SnS2 powder, which the researchers also measured electrochemically, was found to have less catalytic CO2 reduction activity.
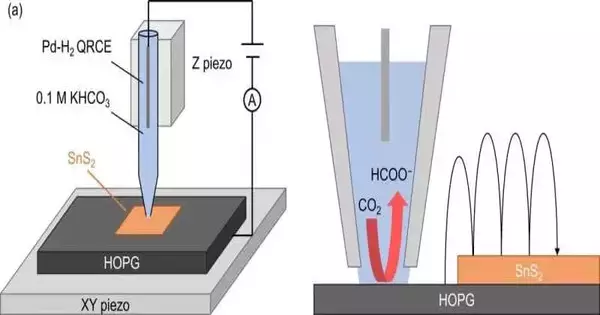
The researchers utilized a technique known as scanning electrochemical cell microscopy (SECCM) in order to comprehend where the catalytically active sites are located in SnS2 and the reason why the 2D material performs better than the bulk compound. SECCM is utilized as a nanopipette to frame the meniscus-shaped nanoscale electrochemical cell for the surface reactivity detection test in the example. The measurements demonstrated that the SnS2 sheet’s entire surface, not just the structure’s “terrace” or “edge” features, is catalytically active. This likewise makes sense of why 2D SnS2 has improved action compared with mass SnS2.
Computations gave further insight into the compound responses at play. In particular, the development of formic corrosive was affirmed as a vivaciously great response pathway while utilizing 2D SnS2 as impetus.
The findings of Takahashi and colleagues represent a significant advancement in the utilization of 2D electrocatalysts in electrochemical CO2 reduction processes. “These findings will provide a better understanding and design strategies for metal dichalcogenide-based 2D electrocatalysis for electrochemical CO2 reduction to produce hydrocarbons, alcohols, fatty acids, and olefins without by-products,” the researchers write.
Background: Materials of the type MX2 consist of two-dimensional (2D) metal dichalcogenide sheets, also known as monolayers. In these materials, M stands for a metal atom like molybdenum (Mo) or tin (Sn), and X stands for a chalcogen atom like sulfur (S). The structure can be depicted as a layer of X atoms layered on top of a layer of M atoms layered on top of another layer of X atoms. Due to their extreme thinness, 2D metal dichalcogenides are referred to as members of the so-called class of 2D materials, which also includes graphene. 2D materials normally have unexpected properties in comparison to their mass (3D) partners.
The electrocatalytic activity of 2D metal dichalcogenides in the hydrogen evolution reaction (HER), a chemical reaction that produces hydrogen, has been the subject of research. However, Yasufumi Takahashi and colleagues from Kanazawa University have now discovered that the 2D metal dichalcogenide SnS2 does not possess any HER catalytic activity; instead, it acts as a catalyst for the electrochemical conversion of carbon dioxide (CO2) into formic acid, which is an extremely important property for implementing CO2 footprint reduction strategies.
More information: Yusuke Kawabe et al, 1T/1H-SnS2 Sheets for Electrochemical CO2 Reduction to Formate, ACS Nano (2023). DOI: 10.1021/acsnano.2c12627