New UMBC-drove research in Boondocks in Microbial science proposes that infections are utilizing data from their current circumstances to “choose” when to hold on inside their hosts and when to duplicate and burst out, killing the host cell. The work has suggestions for antiviral medication improvement.
The new paper says an infection’s capacity to detect its current circumstances, including components created by its host, adds “one more layer of intricacy to the viral-host connection,” says Ivan Erill, teacher of organic sciences and senior creator of the new paper. At this moment, infections are taking advantage of that capacity to their advantage. Yet, later on, he says, “We could take advantage of it to our burden.”
Not an incident
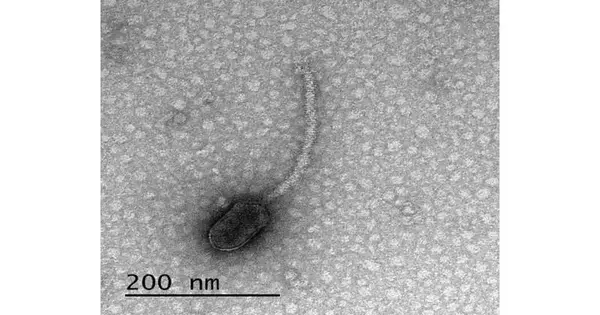
The new review zeroed in on bacteriophages—infections that taint microbes, frequently alluded to as “phages.” The phages in the review can contaminate their hosts when the bacterial cells have unique limbs, called pili and flagella, that help the microorganisms move and mate. The microbes produce a protein called CtrA that controls when they create these limbs. The new paper shows that numerous limb subordinate phages have designs in their DNA where the CtrA protein can join, called restricting locales. Erill says that a phage having a limiting site for a protein created by its host is strange.
“We propose that the phages are monitoring CtrA levels, which rise and fall over the cell’s life cycle, to determine when the swarmer cell is becoming a stalk cell and a factory of swarmers, and at that point, they burst the cell since there will be many swarmers around to infect.”
Ivan Erill, professor of biological sciences
It’s really amazing. Erill was the paper’s most memorable creator Through itemized genomic examination, Elia Mascolo, a Ph.D. understudy in Erill’s lab, found that these limiting locales were not novel to a solitary phage, or even a solitary gathering of phages. Various phages had CtrA-restricting regions, but they generally expected their hosts to have pili and flagella to contaminate them.It couldn’t be an incident, they chose.
The capacity to screen CtrA levels “has been created on various occasions all through development by various phages that taint various microbes,” Erill says. When remotely related species show a comparable quality, it’s called “merged development” — and it demonstrates that the characteristic is certainly helpful.
Timing is everything.
One more kink in the story: the main phage where the examination group recognized CtrA restricting locales taints a specific gathering of microbes called Caulobacterales. Caulobacterales are particularly focused on the gathering of microbes since they exist in two structures: a “swarmer” structure that swims around openly, and a “followed” structure that joins to a surface. The swarmers have pili/flagella, and the stalks don’t. In these microbes, CtrA likewise directs the phone cycle, deciding if a phone will be isolated equally into two business as usual cell types or gap unevenly to create one swarmer and one tail cell.
Since the phages can taint swarmer cells, it’s to their greatest advantage just to burst out of their host when there are numerous swarmer cells free to contaminate. By and large, caulobacterales live in unfortunate conditions, and they are extremely fanned out. Yet, when they track down a decent pocket of microhabitat, they become “followed cells” and multiply, Erill says, at last creating huge numbers of swarmer cells.
Thus, “We guess the phages are checking CtrA levels, which go all over during the existence pattern of the phones, to sort out when the swarmer cell is turning into a tail cell and turning into a plant of swarmers,” Erill explains, “and by then, they burst the phone, since there will be numerous swarmers close by to taint it.”
Tuning in
Sadly, the strategy to demonstrate this speculation is very serious and very troublesome, so that wasn’t essential for this most recent paper — despite the fact that Erill and partners desire to handle that inquiry later on. In any case, the examination group sees no great reason for the expansion of CtrA restricting locales on such countless different phages, all of which require pili/flagella to taint their hosts. What is really intriguing, they note, are the ramifications of infections that taint different creatures — even people.
“All that we are familiar with phages, each and every developmental system they have created, has been displayed to mean infections that taint plants and creatures,” he says. “It’s very nearly guaranteed. So assuming phages are tuning in on their hosts, the infections that influence people will undoubtedly be doing likewise. “
There have been a few other documented instances of phages testing their current circumstances in intriguing ways, but none involve so many different phages using a similar system against so many bacterial hosts.
This new examination is the “main wide degree show that phages are tuning in on what’s happening in the cell, for this situation regarding cell advancement,” Erill says. Yet, more models are coming, he predicts. As of now, individuals from his lab have begun searching for receptors for other bacterial administrative atoms in phages, he says — and they’re tracking them down.
New helpful roads
The vital focus point from this exploration is that “the infection is utilizing cell intel to decide,” Erill says, “and assuming it’s going on in microbes, it’s very likely occurring in plants and creatures, since, supposing that a developmental system seems OK, advancement will find it and take advantage of it.”
For instance, to upgrade its system for endurance and replication, a creature infection should understand what sort of tissue it is in or the way that hearty the host’s safe reaction is to its disease. While it may be unsettling to consider all the data infections could amass and possibly use to make us sicker, these revelations also pave the way for new treatments.
“In the event that you are fostering an antiviral medication, and you realize the infection is tuning in on a specific sign, then perhaps you can trick the infection,” Erill says. In any case, that is a few stages away. For the present, “We are simply beginning to acknowledge how effectively infections have eyes on us—how they are checking what’s happening around them and pursuing choices in view of that,” Erill says. “It’s entrancing.”
More information: Elia Mascolo et al, The transcriptional regulator CtrA controls gene expression in Alphaproteobacteria phages: Evidence for a lytic deferment pathway, Frontiers in Microbiology (2022). DOI: 10.3389/fmicb.2022.918015
Journal information: Frontiers in Microbiology