In a review distributed in the Diary of the American Substance Society, scientists seem to have found a method for delivering a genuine design of the uncommon, but normally happening, hostile HIV compound Lancilactone C from beginning to end.
Its non-cytotoxicity in warm-blooded creatures could make this triterpenoid an optimal contender for treating helplessness, assuming its organic action were clear and in the event that it were plentiful in nature.
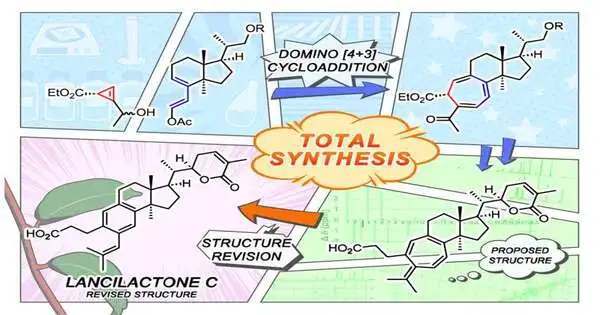
Presently, an exploration group at Kyoto College has prevailed with regards to making a domino-like blend of Lancilactone C’s one-of-a kind seven-membered ring structure.
“Our engineered technique uncovered that the proposed design of Lancilactone C was at first mistaken,” says Chihiro Tsukano of Kyoto College’s Master’s-level college of Agribusiness. “Be that as it may, we effectively got its actual design from our unearthly information and comprehension of its biosynthesis.”
Notwithstanding this disclosure, Tsukano understood that the electrocyclization—aa reworking response in natural science—uutilized in the complete blend likewise happens in biosynthesis.
Unexpectedly, it remains a secret whether the proposed structure containing an unsaturated seven-membered ring could exist in nature as a simple or identical compound and what it could mean for the outflow of organic action.
Tsukano’s group used the domino-like response to empower the complete combination of lancilactones and related triterpenoids. This result has propelled the group to further their exploration of compound designs, prompting the conceivable advancement of novel antivirals.
The unending circle of required prescriptions and multi-drug treatments frequently corresponds with lower personal satisfaction for financially troubled patients.
“Our engineered technique for Lancilactone C, with its known adequacy, may prompt a less risky enemy of HIV drugs,” finishes up Tsukano.
More information: Hidetaka Kuroiwa et al, Total Synthesis and Structure Revision of (+)-Lancilactone C, Journal of the American Chemical Society (2023). DOI: 10.1021/jacs.3c04124