Rare genetic variations may be associated with one of the general mechanisms underlying age-related macular degeneration (AMD), a common cause of vision loss in older adults, according to a study from the National Eye Institute (NEI).
The variations produce abnormal proteins that change the stability of the membrane attack complex (MAC), which could lead to a long-lasting inflammatory response in the retina. The research, which was published in the journal iScience, suggests that MAC may be a useful therapeutic target to delay or stop the progression of AMD. A portion of the National Institutes of Health is NEI.
Although there are numerous genetic variations that can either increase or decrease a person’s risk of developing AMD, each of these genetic variations only makes a small contribution to the disease.
“Only a few studies have specifically identified protein abnormalities that can lead to AMD, even though there are several genetic variations that impact risk for AMD.”
Anand Swaroop, Ph.D., chief of NEI’s Neurobiology,
Anand Swaroop, Ph.D., set out to identify genetic variations—and proteins—that have a clear connection to the condition. D., the lead author of the study and director of NEI’s Neurobiology, Neurodegeneration, and Repair Laboratory, worked with Michael Klein, M.D., a renowned AMD specialist at the Oregon Health Sciences University (OHSU), Portland. Klein has gathered clinical data from hundreds of patients as well as families with many members who have AMD.
Swaroop, Klein, and associates searched for families carrying extremely rare variants that cause AMD, where the effect of the variant on the gene is very strong and where the variant directly affects protein structure and function. This kind of uncommon variant can show the disease’s underlying cause.
Even though there are numerous genetic variations that influence the risk of developing AMD, only a small number have directly linked the disease to changes in specific proteins, according to Swaroop.
We discovered two proteins that may be directly responsible for the pathology of AMD in affected patients by examining large families with ultra-rare variants that closely correlate with disease across generations. Potential targets for upcoming medications include these proteins. “While some treatments are currently available to slow vision loss in people with the wet form of AMD, there is currently no cure for the condition and no treatment for the majority of patients.
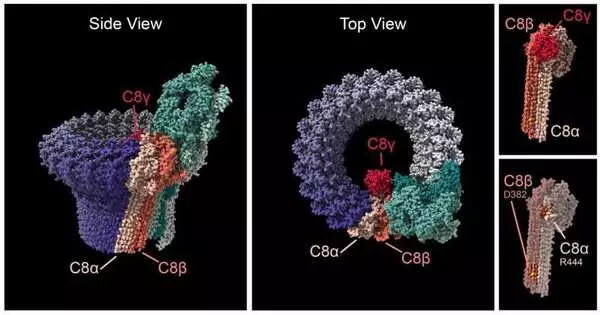
Swaroop, Klein, and colleagues discovered that individuals with AMD have mutations in either of the two C8-alpha or C8-beta proteins, which make up one end of the MAC, in four families. The team discovered that the variations from the four AMD families all had an impact on the C8 proteins’ capacity to bind to one another, which may change how MAC functions in the retina of the eye.
The C8 proteins close a circular pore that is created by MAC at one end, allowing ions to pass through cell membranes. The ‘complement cascade,’ a component of the immune system that aids in the body’s defense against pathogens, ends at this pore. Although it was initially believed that the only purpose of MAC was to enter bacterial cell membranes and eradicate the pathogen, more recent research has revealed that MAC has a complex role in controlling inflammatory processes in tissues like the retina.
C8 proteins and other proteins found higher up in the complement cascade may play a role in AMD, according to genetic information from NEI’s Age Related Eye Disease Studies. As the last step in the complement cascade, MAC, variants affecting any of the complement proteins may funnel down to change MAC function. According to the researchers, either an excessive amount or a deficiency of stable MAC in the retina may cause destructive inflammation, which in turn promotes the progression of AMD.
“We think that targeting MAC may be a more effective strategy to control AMD,” Swaroop said. “Mac is the end of the immune system’s complement pathway, and there’s such a strong link between these rare variants and disease,” he added. “With a small molecule medication, we may be able to regulate how strongly MAC drives inflammation and, as a result, slow down AMD progression.”.
More information: Anand Swaroop et al, Ultra-rare complement factor 8 coding variants in families with age-related macular degeneration, iScience (2023).