Planning a battery is a three-section process. You want a positive cathode, you want a negative terminal, andticriticallye,you want an electrolyte that works with the two anodes.
An electrolyte is the component of a battery that transports charge-carrying particles between the battery’s two cathodes, allowing the battery to charge and discharge.For the present lithium-particle batteries, electrolyte science is somewhat obvious. For people in the future of batteries being created all over the planet and at the U.S. Department of Energy’s (DOE) Argonne Public Lab, in any case, the topic of electrolyte configuration is totally open.
“While we have a specific idea for electrolytes that will work with current business batteries, for past lithium-particle batteries, the planning and improvement of various electrolytes will be critical,” said Shirley Meng, chief researcher at the Argonne Cooperative Place for Energy Storage Science (ACCESS) and professor of sub-atomic design at The University of Chicago’s Pritzker School of Atomic Designing.
“While we are committed to a certain concept for electrolytes that will work with today’s commercial batteries, the design and development of various electrolytes will be critical for beyond-lithium-ion batteries,”
Shirley Meng, chief scientist at the Argonne Collaborative Center for Energy Storage Science (ACCESS)
“Electrolyte improvement is one key to the headway we will accomplish in making these less expensive, longer-enduring, and more impressive batteries a reality and making one significant stride towards proceeding to decarbonize our economy.”
In another paper distributed in Science, Meng and partners spread out their vision for electrolytes in the future of batteries.
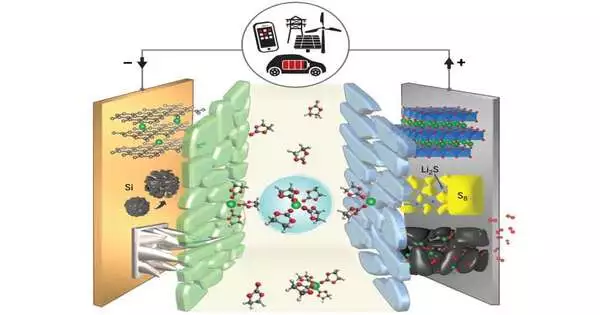
According to Meng, even slightly smaller takeoffs from the current batteries will necessitate a rethinking of the electrolyte configuration.Changing from a nickel-containing oxide to a sulfur-based material as the primary constituent of a lithium-particle battery’s positive cathode could yield huge execution benefits and lessen costs in the event that researchers can sort out some way to rejigger the electrolyte, she said.
For other past lithium-particle battery sciences, such as battery-powered sodium-particle or lithium-oxygen, researchers will also need to give extensive regard to the subject of the electrolyte.
One central point that researchers are thinking about in the advancement of new electrolytes is the way they will generally frame a delegate layer called an interphase, which bridles the reactivity of the cathodes. “Interphases are vitally critical to the working of a battery since they control how specific particles stream into and out of the cathodes,” Meng said. “Interphases capability like a door to the remainder of the battery; in the event that your entryway doesn’t work as expected, the specific vehicle doesn’t work.”
The group’s short-term objective is to select electrolytes with the right compound and electrochemical properties to enable the ideal development of interphases at both the battery’s positive and negative anodes. At last, in any case, scientists accept that they might have the option to foster a gathering of strong electrolytes that would be steady at outrageous (both high and low) temperatures and empower batteries with high energy to have significantly longer lifetimes.
“A strong state electrolyte for an all-strong battery will be a unique advantage,” said Venkat Srinivasan, head of ACCESS, delegate overseer of the Joint Place for Energy Stockpiling Exploration, and co-creator of the paper. “The way into a strong state battery is a metal anode, yet its exhibition is presently restricted by the development of needle-like designs called dendrites that can short out the battery.” “By finding a strong electrolyte that forecloses or hinders dendrite development, we might have the option of understanding the advantages of some truly thrilling battery sciences.”
To speed up their hunt for electrolytes, researchers have used cutting-edge visualization and artificial reasoning (simulated intelligence) to thoroughly examine a lot more potential competitors, accelerating what had previously been a slow and meticulous course of lab union.
“Elite execution figuring and man-made reasoning are permitting us to recognize the best descriptors and qualities that will empower the customized use of different electrolytes for explicit purposes,” Meng said. “Rather than taking a gander at a couple dozen electrolyte prospects a year in the lab, we’re checking out a huge number with the help of calculation.”
“Electrolytes have billions of potential mixes of parts—ssalts, solvents, and added substances—tthat we can play with,” Srinivasan said. “To make that number into something more sensible, we’re starting to truly utilize the force of man-made intelligence, AI, and robotized labs.”
The mechanized labs to which Srinivasan alluded will integrate a robot-driven trial system. Similarly, machines can perform unassisted, painstakingly refined, and aligned tests to ultimately determine which combination of parts will shape the ideal electrolyte.”Robotized disclosure can decisively increase the force of our exploration because machines can work nonstop and reduce the possibility of human error,” he said.
Meng, Srinivasan, and Armed Forces Exploration Lab researcher Kang Xu examine the electrolyte challenge in a paper titled “Planning better electrolytes,” which showed up in Science on Dec. 8.
More information: Y. Shirley Meng et al, Designing better electrolytes, Science (2022). DOI: 10.1126/science.abq3750
Journal information: Science