The human safe framework is a profoundly intricate organization of cells, signs, and reactions that is firmly managed to guarantee that the body can ward off disease without harming its own tissues. Presently, scientists from Japan report another manner in which the safe framework shields lung tissue from viral diseases.
In a review distributed in Cell Reports, scientists from the Nara Foundation for Science and Innovation (NAIST) have uncovered that antigen-explicit executioner lymphocytes (CD8+ immune system microorganisms) quickly grow in the lungs when they experience antigen-introducing alveolar macrophages (AMs) to safeguard against viral disease.
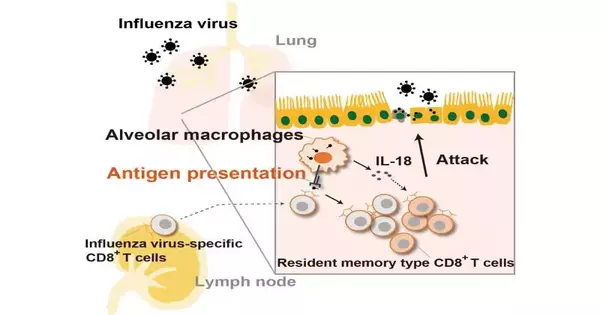
CD8+ lymphocytes give defensive resistance against diseases associated with respiratory infections, like flu. an infection (IAV) and the extremely intense respiratory disorder COVID-2 (SARS-CoV-2) by killing tainted cells. To target the right cells for killing, gullible CD8+ lymphocytes should be exposed to antigen-introducing cells (APCs), which intervene in the take-up of infection-tainted cells and present their antigens in a process known as cross-show.The prepared CD8+ lymphocytes then clonally grow and separate into effector or enduring antigen-explicit memory white blood cells.
“Multiple cell types can present antigen to CD8+ T lymphocytes in the lungs, but the involvement of tissue-resident macrophages in this process is unknown,”
Takumi Kawasaki, lead author of the study.
“Various cell types can introduce antigen to CD8+ lymphocytes in the lungs, albeit the job of tissue-occupant macrophages in this cycle is hazy,” makes sense to Takumi Kawasaki, the lead creator of the review. “AMs are the first cells in quite a while that experience irresistible materials, natural particles, surfactants, and passing on cells, and they are significant for the host’s defense against bacterial and parasitic disease, so we thought that they were likewise significant in safeguarding against respiratory infection contamination.”
To test this, the analysts investigated the systems by which APCs educate antigen-explicit CD8+ lymphocytes in the lungs. In the first place, mice were prepared by inoculation with a particular antigen or disease using IAV, and afterward they were exposed to optional vaccination or re-contamination.
“We demonstrated that antigen-introducing AMs present breathed in antigen to memory CD8+ lymphocytes, and that this resulted in a rapid expansion of antigen-explicit CD8+ white blood cells in the lungs,” says Taro Kawai, senior author of the study.
Besides, the analysts found that AMs help foster a memory-type cell population by creating interleukin 18. Critically, the organization of antigen-stacked AMs in mice incited the expansion of memory-type CD8+ lymphocytes.
“This system might work on the adequacy of CD8+ lymphocyte subordinate cell resistance,” says Kawai.
Considering that the lung is a significant tissue for IAV and SARS-CoV-2 disease, the discoveries from this study in regards to the system of lung-occupant memory CD8+ cell extension are supposed to prompt the improvement of new immunizations that incite cell resistance. In the future, infection-specific antigen-introducing AMs could be delivered as a type of “cell relocation immunization.”
More information: Takumi Kawasaki et al, Alveolar macrophages instruct CD8+ T cell expansion by antigen cross-presentation in lung, Cell Reports (2022). DOI: 10.1016/j.celrep.2022.111828
Journal information: Cell Reports