A huge report driven by Lund College in Sweden has demonstrated the way that individuals with Alzheimer’s sickness can now be recognized before they experience any side effects. It is now also possible to predict who will fall apart over the next couple of years.The review is distributed in Nature Medicine and is convenient considering the advancement of new medications for Alzheimer’s disease.
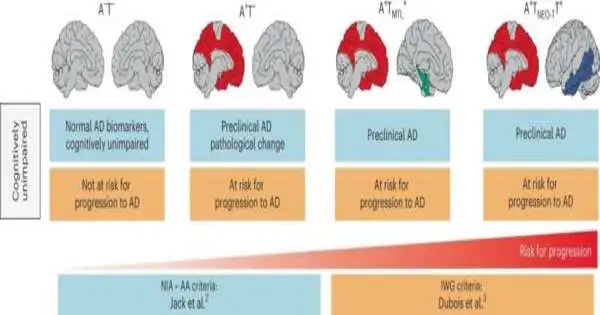
It has been known for quite some time that there are two proteins connected to Alzheimer’s: beta-amyloid, which structures plaques in the mind, and tau, which at a later stage collects inside synapses. Raised levels of these proteins in combination with mental weakness have recently been framed as the reason for diagnosing Alzheimer’s.
“Changes in the brain occur between ten and twenty years before the patient exhibits any obvious symptoms, and it is only after tau begins to spread that nerve cells die and the patient exhibits the first cognitive impairments. This is why Alzheimer’s disease is so difficult to detect in its early stages.”
Oskar Hansson, senior physician in neurology at Skåne University Hospital
“Changes happen in the mind somewhere between 10 and 20 years before the patient encounters any reasonable side effects, and it is just when tau starts to spread that the nerve cells bite the dust and the individual being referred to encounters the primary mental issues.” To this end, Alzheimer’s is so challenging to analyze in its early phases, “which makes sense,” says Oskar Hansson, senior doctor in nervous system science at Skne College Emergency Clinic and teacher at Lund College.
He has now driven an enormous worldwide examination effort that was completed with 1,325 members from Sweden, the US, the Netherlands, and Australia. The members showed no mental weakness toward the start of the review. By utilizing PET outputs, the presence of tau and amyloid in the members’ minds could be imagined.
Individuals in whom the two proteins were found were viewed as having a 20–40 times higher chance of fostering the sickness at follow-up a couple of years after the fact, contrasted with the members who had no natural changes.
“When both beta-amyloid and tau are present in the cerebrum, it can no longer be viewed as a gamble factor, but rather a conclusion.””A pathologist who inspects tests from a cerebrum like this would promptly determine the patient to have Alzheimer’s,” says Rik Ossenkoppele, who is the primary creator of the review and is a senior scientist at Lund College and Amsterdam College Clinical Center.
He makes sense of the fact that Alzheimer’s specialists have a place with two ways of thinking—on the one hand, the individuals who accept that Alzheimer’s sickness can’t be analyzed until mental impedance starts. There is likewise the gathering that he and his partners have a place withtwo ways of thinking—on the one hand, the individuals who accept that Alzheimer’s sickness can’t be analyzed until mental impedance starts. There is likewise the gathering that he and his partners have a place with—those who say that a determination can be founded simply on science and what you can find in the mind.
“You can, for instance, contrast our outcomes with those of prostate disease. “On the off chance that you perform a biopsy and find disease cells, the conclusion will be malignant growth, regardless of whether the individual being referred to has not yet developed side effects,” says Rik Ossenkoppele.
As of late, positive outcomes have arisen in the clinical preliminaries of another medication against Alzheimer’s, Lecanemab, which has been assessed in Alzheimer’s patients. In view of this, the review from Lund College is especially fascinating, say the analysts:
“In the event that we can analyze the illness before mental difficulties show up, we may ultimately have the option to utilize the medication to dial back the sickness at the beginning phase.” When combined with actual work and good nutrition, one has a better chance of avoiding or reducing future mental distress. “However, more research is required before treatment can be recommended for individuals who have not yet developed cognitive decline,” Oskar Hansson concludes.
More information: Rik Ossenkoppele et al, Amyloid and tau PET-positive cognitively unimpaired individuals are at high risk for future cognitive decline, Nature Medicine (2022). DOI: 10.1038/s41591-022-02049-x
Journal information: Nature Medicine