Changes in their spatial design, for example, cause collapsing errors (misfolding) in proteins or peptides.This is the aftereffect of momentary deviations in the compound organization of the biomolecules. Specialists at the Karlsruhe Institute of Technology (KIT) have fostered a basic and successful strategy for recognizing such misfolding at the beginning phase of the sickness. Misfolding is uncovered by the construction of dried buildup from protein and peptide arrangements. The strategy includes investigating micrographs with brain organizations and has a prescient exactness of over 95%. The outcomes have been published in Advanced Materials.
The biochemical construction of proteins and peptides determines their natural capabilities. There are numerous signs that even momentary primary or spatial changes can advance the improvement of illnesses. Numerous neurodegenerative infections have been credited to the misfolding of proteins and peptides that is brought about by such changes. Amyloid beta (A42) peptides assume a critical part in Alzheimer’s sickness; they contrast in a solitary amino corrosive buildup and address genetic freaks of Alzheimer’s illness.
“The stain patterns had a prediction accuracy of more than 99 percent and led to the classification of eight mutations in addition to being distinctive and reproducible.”
Lahann, author of the study,
Up to this point, there has not been a straightforward and exact strategy for anticipating changes in proteins. KIT’s Institute of Functional Interfaces (IFG), an examination group led by Professor Jörg Lahann, has fostered a strategy for recognizing misfolding through the design of dried protein and peptide arrangements. “The stain designs were trademark and reproducible as well as resulted in a characterization of eight transformations with a prescient precision of over close to 100%,” said Lahann, creator of the review, in portraying the outcomes. The data showed that critical data about the essential and optional designs of peptides can be gathered from the stains abandoned by drying drops of peptide arrangement on a strong surface.
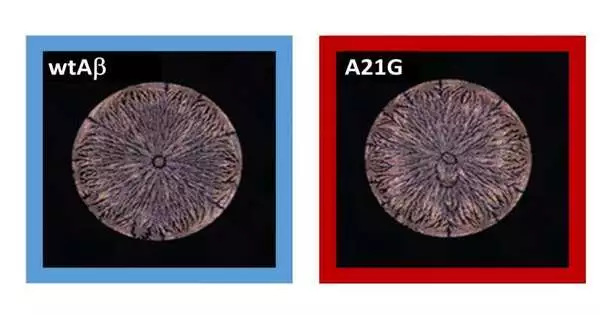
Stain designs as careful peptide fingerprints
The protein and peptide arrangements are definitively put on glass slides by a mechanized pipetting framework to guarantee controlled and reproducible outcomes. The surfaces of the slides were prepared ahead of time with a hydrophobic polymer covering. To examine the perplexing stain designs from the dried drops, the analysts procured pictures utilizing polarization microscopy. The pictures were then examined with profound learning brain organizations.
“Since the designs are practically the same and hard to recognize with the unaided eye, it was certainly unexpected that the brain networks were so successful,” explains Lahann about the outcomes. “The stain examples of amyloid beta peptides act as definite fingerprints that mirror the primary and spatial personality of a peptide.” This innovation empowers the recognizable proof of Alzheimer variations with the greatest goal inside a couple of moments, as per Lahann.
Simple example arrangement communicates quick analyses
The outcomes propose that a strategy as basic as drying a bead of peptide arrangement on a strong surface can act as a marker for minute contrasts in the essential and optional designs of peptides. “Versatile and exact identification strategies for the delineation of conformational and underlying protein changes are earnestly required to decipher the obsessive marks of infections like Alzheimer’s and Parkinson’s,” says Lahann.
Likewise, a somewhat basic strategy requires no intricate planning of tests and, in this manner, empowers a straightforward and patient-accommodating conclusion. Moreover, the strategy has extraordinary potential for different applications in clinical diagnostics and in the sub-atomic identification of illnesses.
More information: Azam Jeihanipour et al, Deep‐Learning‐Assisted Stratification of Amyloid Beta Mutants Using Drying Droplet Patterns, Advanced Materials (2022). DOI: 10.1002/adma.202110404