Effective purging cycles of different pollutants from air and water are important to support life on the planet. To this end, carbon materials have for some time been utilized for aerating, isolating, and eliminating unsafe anion pollution by adsorption. Up to this point, the itemized system by which carbon purges water has stayed a secret. Also, it isn’t known whether the fluid arrangement adsorbed on the carbon material is acidic, basic, or unbiased.
To address these holes, analysts led by Dr. Takahiro Ohkubo, academic partner in the Branch of Science, Staff of Innate Science and Innovation, Okayama College, Japan, explored the key system by which anions are adsorbed via carbon nanopores.
In a new article made accessible online on September 16, 2022 and distributed in the Diary of Colloid and Connection Point Science, the scientists report utilizing Raman spectroscopic devices to look at the adsorption of nitrate particles by the tube-shaped pore of single-walled carbon nanotubes (SWCNT).
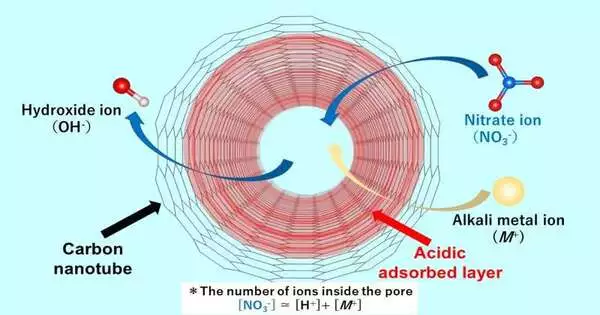
“The acidic layer in the pore can strongly adsorb the nitrate anion species due to both tight confinement by the pore and strong interaction between the layer and the anion.”
Dr. Takahiro Ohkubo, associate professor in the Department of Chemistry
With regards to unraveling the system of acidic layer arrangement close to the pore walls, Dr. Ohkubo and his partners prevailed. It would seem, when a fluid arrangement containing particles enters the carbon material, regardless of whether the watery arrangement is unbiased, an acidic fluid layer containing protons is shaped that keeps a steady state. Remarking on the oddity and key nature of their work, Dr. Ohkubo said, “until now, there have been no reports showing the presence of acidic adsorption layers shaped inside nanotubes of carbon materials.”
The research group, which also included Dr. Nobuyuki Takeyasu, an academic partner on a similar staff at Okayama College, discovered that the acidic layer works with effective adsorption of the negatively charged nitrate anion pollution, where the adsorbed measure of nitrate particles is significantly greater than that of the cations, or the strongly charged gatherings.Also, hydroxide particles are created as counter-particles. The anions present in the mass arrangement are traded with the hydroxide particles in the SWCNT, making the fluid arrangement basic.
The group analyzed anion adsorption utilizing a few salt metal nitrates, including lithium nitrate, sodium nitrate, rubidium nitrate, and cesium nitrate arrangements. They observed that more nitrate particles are adsorbed than metal particles. The amount of proton adsorption was nearly identical regardless of the type of salt metal particle used.(Dr. According to Ohkubo, “The acidic layer in the pore can firmly adsorb the nitrate anion species due to both solid control by the pore and the solid connection between the layer and the anion.”
The discoveries are definitely significant steps towards planning and creating carbon nanotubes reasonable for particle adsorption and purging of water and air. The system of purging set out in this examination is a clever model that makes sense of the alkalinity of the fluid arrangement medium, which has until now been a secret. The analysts see that the discoveries of their concentrate firmly highlight the need to kill water before use when ionic pollutants are caught via carbon materials.
One more striking commitment of this study is that it shows that the nanomaterial connection point is a clever compound response field, which could direct further tests. This work takes how we might interpret the system of anion adsorption via carbon to a higher level, clearing the path for novel carbon nanotubes as effective purifiers.
More information: Takahiro Ohkubo et al, Acidic layer-enhanced nanoconfinement of anions in cylindrical pore of single-walled carbon nanotube, Journal of Colloid and Interface Science (2022). DOI: 10.1016/j.jcis.2022.09.070