Antibiotics are used to kill bacteria that cause illness and disease. They have made significant contributions to human health. Many diseases that used to kill people can now be effectively treated with antibiotics. Some bacteria, however, have developed resistance to commonly used antibiotics.
Antibiotic resistant bacteria are those that are resistant to or killed by antibiotics. In the presence of an antibiotic, they can survive and even multiply. Most pathogenic bacteria can develop resistance to at least some antibiotics. Multi-resistant organisms are bacteria that are resistant to multiple antibiotics (MRO).
While there are many studies that discuss antibiotic resistance genes (ARG) in soil and water environments, there is currently very little research that focuses on ARG in aerial environments. In a recent review, researchers have analyzed current research trends regarding ARG in bioaerosols, including their sources, methods of detection, and implications for the future.
Bioaerosols, which are airborne biological particles that contain viruses, fungal spores, bacteria, and pollen, are important in public health. Because of horizontal gene transfer between the same or similar bacterial bioaerosols, antibiotic resistance (AR) caused by antibiotic resistance genes (ARGs) has the potential to cause global public health crises.
We wanted to know what was driving ARG in bioaerosols. We thoroughly reviewed data on the potential risk factors associated with AR in bioaerosols, their impact on human health, appropriate methodologies, and the most recent technologies and advances in bioaerosol monitoring.
Assistant Professor Dr. Keunje Yoo
AR in soil and water microbes has been extensively studied using cutting-edge molecular and biotechnological methods; however, research into antibiotic-resistant bioaerosols in aerial ecosystems is limited. The difficulty in obtaining sufficient quantities of bioaerosols for analyses is the main issue in studying AR in bioaerosols.
To shed more light, Assistant Professor Dr. Keunje Yoo and his colleague at Korea Maritime and Ocean University compiled existing knowledge on ARG in bioaerosols in a comprehensive review published in Reviews in Environmental Science and Bio/Technology. Dr. Yoo explains the motivation behind this article, saying, “We wanted to know what was driving ARG in bioaerosols. We thoroughly reviewed data on the potential risk factors associated with AR in bioaerosols, their impact on human health, appropriate methodologies, and the most recent technologies and advances in bioaerosol monitoring.”
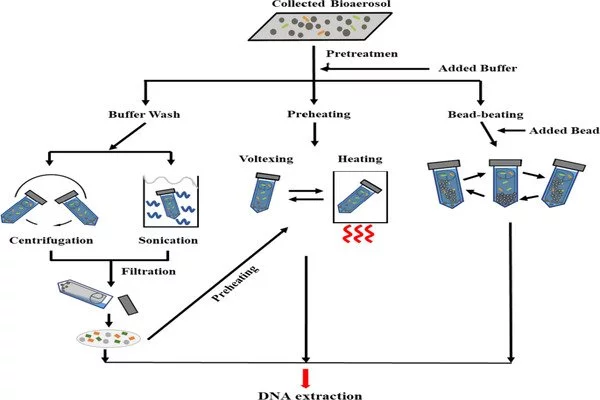
So, what did they discover? Antibiotic-resistant bioaerosols are produced from a variety of sources, which are classified as either indoor or outdoor environments. Hospitals, healthcare facilities, laboratory buildings, and downtown facilities such as offices, markets, universities, and kindergartens are examples of indoor environments. Impaction, impingement, and filtration are the most effective methods for detecting these bioaerosols. To assess the prevalence and levels of ARG in samples, molecular and biotechnological methods such as quantitative real-time PCR (qPCR), high throughput qPCR, and high throughput sequencing techniques are used.
According to the World Health Organization, the spread of AR is one of the top ten global public health concerns confronting humanity. To combat the spread of AR, it is critical to investigate the relationship between human, animal, and environmental microbiomes. “By identifying which bacteria generate and transfer AR and understanding the interaction between host cells, environment, tissues, and pathogens at a molecular level,” Dr. Yoo concludes, “we move a step closer to finding targets in managing AR in the environment.”
Antibiotic resistance is a serious public health problem. It can be prevented by minimizing unnecessary prescribing and overprescribing of antibiotics, the correct use of prescribed antibiotics, and good hygiene and infection control. Some bacteria are naturally resistant to some antibiotics. For example, benzylpenicillin has very little effect on most organisms found in the human digestive system (gut).
Although antibiotics and antifungals save lives, their use can contribute to the development of resistant bacteria. Antimicrobial resistance is accelerated when bacteria and fungi are forced to adapt due to the presence of antibiotics and antifungals. Antibiotics and antifungals kill some germs that cause infections, but they also kill beneficial germs that keep our bodies healthy. Antimicrobial-resistant germs survive and reproduce. Resistance traits in the DNA of these surviving germs can spread to other germs.