Persistent lung infections, chronic wounds, and health-care-associated infections are far more difficult to treat than other types of bacterial infections. This is due to the fact that they are frequently caused by biofilms, which are colonies of microbes—mostly bacteria—that grow together in a self-produced matrix that protects and isolates them from the outside environment.
According to a study published in the journal npj Biofilms and Microbiomes, a new triple-acting antibiotic agent has managed to break through the biofilm extracellular matrix and eliminate more than 50% of the pathogens in one shot.
The study was led by Eduard Torrents, lecturer at the Department of Genetics, Microbiology, and Statistics of the Faculty of Biology and head of the group on Bacterial Infections: Antimicrobial Therapy of the Institute for Bioengineering of Catalonia (IBEC).
This extracellular matrix exacerbates antibiotic resistance, which, according to the World Health Organization (WHO), is one of the most serious threats to global health, because killing bacteria inside the film is up to thousand times more difficult. As a result, biofilm infections are the most important nonspecific mechanism of antimicrobial resistance.
Only using antibiotics to combat these microbes is insufficient. There is a need for tools that can penetrate the extracellular matrix and kill the bacteria inside. The first author of this study, which has reached this milestone, is Nria Blanco-Cabra, a postdoctoral researcher in Eduard Torrents’ group at UB and IBEC. The research was carried out in collaboration with CIDETEC scientists (Basque Country).
With a single dose of our agent achieving such significant biofilm clearance, we predicted that a full course of antibiotics could significantly reduce the burden of these infections, which are extremely difficult to treat.
Eduard Torrents
A triple-acting drug combination
The study focused on the bacteria Pseudomonas areuginosa, a pathogen that often grows in biofilms in the lungs of those patients with cystic fibrosis or chronic obstructive pulmonary disease (COPD), causing persistent infections. “We grew biofilm cultures in vitro, using a technique that resembles to the way in which they exist and grow in nature,” added Torrents.
In clinical practice, these infections are usually treated with an antibiotic called tobramycin. However, its effectiveness is limited by its inability to penetrate the film. This happens because tobramycin, which is positively charged, is neutralized by the extracellular matrix (with a negative charge).
The researchers loaded the antibiotic into negatively charged nanoparticle carriers. This could neutralize the positive charge before the drug reached the biofilm, which enabled it to break the extracellular matrix and kill the bacteria inside. What is more important, these carriers—built by dextran-based single chain nanoparticles—were able to transport up to 40% of the weight of the antibiotic.
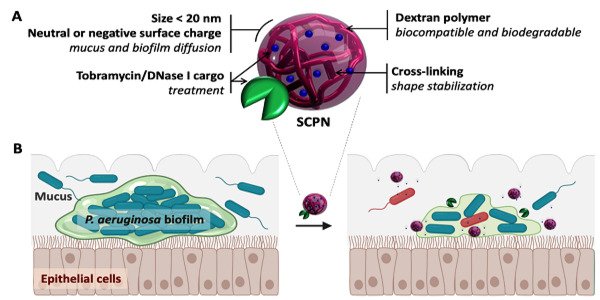
“Many of the previously studied nanotransporters have only been able to sustain a small load of the target compound, which has prevented their clinical use. We managed to overcome this obstacle,” says Torrents.
The antibiotic-loaded nanocarriers were also coated in DNase I, an enzyme. Structural DNA, which is found throughout the extracellular matrix, is one of the compounds that holds bacteria biofilms together. DNase I can break down this “glue,” causing the matrix to loosen and the antibiotic to penetrate deeper into the biofilm.
“By combining an antibiotic with a couple of agents that break up the biofilm, we created a drug that is more powerful than the antibiotic alone in killing the bacteria that lives inside the film,” says Eduard Torrents, the study’s principal investigator.
Using microscopy images, the researchers found that the new agent had not only dissolved the structural DNA in the extracellular matrix, but also that it was acting on and killing the bacteria inside. With just a single application, they reduced the bacterial biomass by more than half.
“With a single dose of our agent achieving such significant biofilm clearance, we predicted that a full course of antibiotics could significantly reduce the burden of these infections, which are extremely difficult to treat.”
In future clinical trials, this agent could be given in multiple doses, as is common with antibiotics. The following step will be to work on clinical validation of this system. Its commercialization would be a significant step forward in the treatment of biofilm infections, which currently cost the global economy $4 billion per year.