Antibodies are important for more than just treating infections and tumors. Now and again, be that as it may, the safe response they trigger can be areas of strength for excessively winding up causing more harm, for instance, on account of individuals contaminated with Coronavirus. As reported in the journal Nature Immunology by Prof. Dr. Falk Nimmerjahn from Friedrich-Alexander University and two of his colleagues from the Netherlands and the UK, problems like these can frequently be avoided by fine-tuning antibodies.
The researcher at FAU is conducting research in his lab on immunoglobulin G, or IgG for short. IgG protects humans and animals from infection for a long time. These biomolecules, which are often utilized in present-day medication, comprise two long and two short chains of proteins that connect together to frame a Y-shaped structure.
For a long time, exploration and medication have zeroed in on the two top parts of this Y for a good explanation: Similar to a key in a lock, the two ends form a type of pocket in which smaller structures on the surface of bacteria and other pathogens fit.
“We have frequently observed cells working together, with granulocytes taking on a suicidal role,”
Prof. Dr. Falk Nimmerjahn from Friedrich–Alexander University.
Key-secure guidelines in a resistant framework
Very much like a locksmith can deliver a lot of various locks and the matching keys by just making a couple of slight changes, the invulnerable framework additionally creates a lot of various designs at the closures of immunoglobulins that match a lot of various microorganisms. After contamination with a particular bacterium or infection, these IgG made during the safe response stay on the lookout inside the body for quite a while and can respond very quickly on account of a restored disease.
Immunoglobulin attaches to the pathogen and marks it for other immune specialists in the immune system if the key fits the lock. The counteracting agent marks growth cells or microbes to make them stand apart from the gigantic amounts of cells and innocuous microorganisms that course all through the body and take on significant capabilities in the collections of people and creatures.
Using genetic glue to fight bacteria
After this stage has been completed successfully, the Y-shaped IgG’s backbone comes into play. Falk Nimmerjahn is currently conducting a thorough investigation into this backbone. Macrophages, executioner cells, and granulocytes take over in the end period of the fight against contamination. ” Falk Nimmerjahn explains, “We have frequently observed cells working as a team, with granulocytes taking on a suicidal role.”
These cells explode, releasing their relatively sticky genetic material from their core, drawn in by the antibody that has identified its target. This substance contains the harmful bacteria that the IgG previously identified.
These potentially lethal microorganisms are now helpless and easy prey for the macrophages that have also been drawn and are now able to consume the bacteria that the antibodies have identified and marked. Notwithstanding, the macrophages are frequently rather forceful and act with little thought of potential outcomes.
Collateral damage, such as the release of oxygen radicals and other harmful products that would normally be rendered harmless, is accepted as inevitable when time is running out in the race between life and death. For most patients, this is of no consequence. The most important thing is to survive, and any damage that happens should be fixable later.
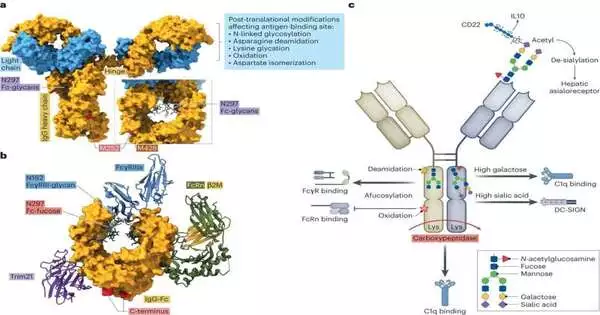
Small posttranslational modifications to the immunoglobulin backbone that are made after the antibody is made are one factor that influences the immune response. This includes, for instance, tiny sugar molecules that are attached to the immunoglobulin’s backbone. They appear to be essential in the fine-tuning of the immune response. According to Falk Nimmerjahn, “if the appropriate components are missing, it makes the immune reaction much more severe.”
However, if a viral infection has already severely damaged tissue, this could result in death. In the event that the control component on the foundation of the immunoglobulin is changed in accordance with just connecting a little sugar and thusly prompting areas of strength for a, that might make perilously extreme harm to an organ that is as of now extended as far as possible, like the lung in the case of a viral disease.
As per Falk Nimmerjahn, “the creature in this manner changes its control components precisely.” In situations like this one, the control mechanisms are set to start a weak reaction with a lot of sugar chains. If we want to improve and increase patients’ tolerance of antibodies used to treat tumors and autoimmune diseases, it is essential to acquire precise knowledge of this antibody tuning within the context of an immune response.
More information: Falk Nimmerjahn et al, Effect of posttranslational modifications and subclass on IgG activity: from immunity to immunotherapy, Nature Immunology (2023). DOI: 10.1038/s41590-023-01544-8