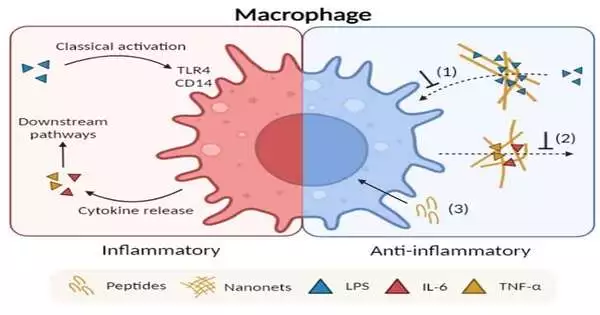
Pharmacists at the National University of Singapore (NUS) have created multifunctional synthetic peptide nanonets to treat bacterial infection-induced inflammation. By simultaneously capturing bacterial endotoxins and pro-inflammatory cytokines, this is accomplished.
Endotoxemia is characterized by the presence of endotoxins in the blood. During systemic infections, gram-negative pathogens like E. coli may release these endotoxins. When left uncontrolled, fiery host reactions can bring about broad tissue harm and septic shock, which are related to a high death rate. Due to the complex interactions between pro- and anti-inflammatory mediators, previous research efforts to develop targeted therapies for sepsis have, regrettably, largely failed.
For better management of septic complications, a more recent strategy places an emphasis on multi-cytokine therapy. Antibacterial peptide nanonets may have additional functions for reducing inflammatory responses that are frequently associated with bacterial infections, as demonstrated by a team of researchers led by Associate Professor Rachel Ee from the Department of Pharmacy at NUS.
“Our peptide-based nanonets have demonstrated unique therapeutic potential as a multi-functional biomaterial for holistic sepsis management. We intend to continue optimizing them for clinical usage in the future.”
Associate Professor Rachel Ee from the Department of Pharmacy,
These discoveries are based on the group’s past report on the plan against microbial peptides fit for in situ self-gathering into microorganism-catching nanonets. The nanonets were able to bind and entrap endotoxins released by gram-negative pathogens and inflammation mediators produced by host macrophages, thereby achieving anti-inflammatory activity. Of interest, the cationic nanonets are specifically favorable to incendiary cytokines while negligibly restricting the mitigating cytokines. The group accomplished this positive explicitness by exploiting the general distinction in net charge between these two unique gatherings of cytokines.
The new review is distributed in the diary, Progressed Medical Services Materials.
Additionally, the lipopolysaccharide (LPS)-binding effect restored colistin’s antimicrobial activity against gram-negative pathogens as a last-line treatment. Prominently, this is the primary example revealed of multi-utilitarian peptide nanonets with long-running impacts in reducing the harms of septic complexities at numerous stages. The peptide nanonets’ ability to lower the level of pro-inflammatory cytokines in the bronchoalveolar fluid of endotoxin-inoculated murine models was demonstrated by biological evaluations carried out on an acute lung injury model. The peptide effect that was produced was comparable to that of the control drug dexamethasone.
Prof. Ee said, “Our peptide-based nanonets have shown special restorative potential as a multi-useful biomaterial for all-encompassing administration of sepsis. We hope to keep optimizing them for clinical use in the future.”
More information: Nhan Dai Thien Tram et al, Multifunctional Antibacterial Nanonets Attenuate Inflammatory Responses through Selective Trapping of Endotoxins and Pro‐Inflammatory Cytokines, Advanced Healthcare Materials (2023). DOI: 10.1002/adhm.202203232