As the world is moving towards additional climate-friendly and economic wellsprings of energy, power modules are getting a ton of consideration. The fundamental benefit of energy components is that they use hydrogen, a perfect fuel, and produce just water as a result while creating power. This new and clean source of energy could replace standard lithium-particle batteries, which currently power all cutting-edge electronic gadgets.
Most power modules utilize a Nafion layer—a manufactured polymer-based ionic film—which acts as a water-based proton-directing strong electrolyte. The utilization of water as a proton conduction medium, in any case, poses a significant disadvantage for the energy unit. To be specific, the powerlessness to work appropriately at temperatures above 100°C, the temperature at which water begins to bubble, prompting a drop in proton conductivity. In this way, there is a requirement for new proton guides that can move protons effectively even at such high temperatures.
“Imidazole, a nitrogen-containing organic molecule, has gained appeal as a proton conductor alternative due to its capacity to function even in the absence of water. It does, however, have a lower proton transfer rate than Nafion and melts at 120°C. To address these difficulties, we incorporated six imidazole moieties into a ruthenium (III) ion to create a novel metal complex that functions as a multi-proton carrier and is highly stable at high temperatures.”
Prof. Tadokoro
In a new forward leap, a group of scientists from Japan, led by Prof. Makoto Tadokoro from Tokyo University of Science (TUS), revealed an original imidazole-imidazolate metal complex based high-temperature proton conveyor that shows proficient proton conductivity even at 147 °C. The examination group included Dr. Fumiya Kobayashi from TUS, Dr. Tomoyuki Akutagawa and Dr. Norihisa Hoshino from Tohoku University, Dr. Hajime Kamebuchi from Nihon University, Dr. Motohiro Mizuno from Kanazawa University, and Dr. Jun Miyazaki from Tokyo Denki University.
“Imidazole, a nitrogen-containing natural compound, has acquired prominence as an elective proton guide for its capacity to work even without water.” Nonetheless, it has a lower proton move rate than commonly used Nafion and melts at 120 °C.To beat these issues, we brought six imidazole moieties into a ruthenium (III) particle to plan another metal complex that works as a multi-proton transporter and has high temperature dependability,” makes sense to Prof. Tadokoro when he gets some information about the reasoning behind their review, which was distributed in Chemistry—A European Journal and highlighted on the title page of the diary.

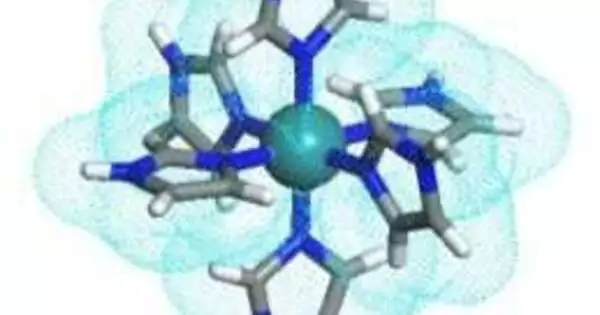
In another review, scientists from Japan have fostered a clever ruthenium (III) particle complex with six imidazole/imidazolate bunches that can work as multi-proton transporters and show high temperature soundness. The top picture portrays the proton transport mode beneath 147 C, which includes individual limited pivots of the six individual imidazole gatherings and proton leaps to other ruthenium (III) buildings. The base picture depicts the proton transport mode above 147 C, where the entire particle goes through a revolution.
The group planned another particle where three imidazole (HIm) and three imidazolate (Im-) bunches were connected to a focal ruthenium (III) particle (Ru3+). The subsequent single sub-atomic precious stone was exceptionally balanced and looked like a “starburst” shape. After examining the proton conductivity of this starburst-type metal intricate, the group found that every one of the six imidazole bunches joined to the Ru3+ particle goes about as a proton transmitter. This made the particle multiple times more powerful than individual HIm particles, which could ship each proton in turn.
The group likewise investigated the component fundamental the high-temperature proton conduction capacity of the starburst particles. They tracked down that at a temperature of more than – 70°C, the proton conductivity came about because of individual restricted pivots of the HIm and Im-gatherings and proton leap to other Ru(III)complexes in the gem by means of hydrogen bonds. At temperatures past 147°C, nonetheless, the proton conductivity emerged from entire particle revolution, which was likewise answerable for the predominant proton conductivity at high temperatures. This turn, affirmed by the group utilizing a procedure called “strong state 2H-NMR spectroscopy,” brought about a conductivity rate three significant degrees bigger (σ = 3.08 × 10-5 S/cm) than that for individual HIm particles (σ = 10-8 S/cm).
The group accepts that their review could go about as another driving guideline for proton-leading strong state electrolytes. The bits of knowledge from their original atomic plan could be utilized to foster new high-temperature proton guides as well as work on the usefulness of the current ones. “Energy units hold the key to a cleaner and greener tomorrow. “Our review offers a guide for working on the presentation of these carbon-impartial energy assets at high temperatures by planning and executing sub-atomic proton guides that can move protons productively at such temperatures,” concludes Prof. Tadokoro.
More information: Makoto Tadokoro et al, Proton Conduction at High Temperature in High‐SymmetryHydrogen‐Bonded Molecular Crystal of Ru(III) Complexes with SixImidazole–Imidazolate Ligands, Chemistry—A European Journal (2022). DOI: 10.1002/chem.202201397