Bacteria, like humans, have a variety of immune systems to defend against diseases such as viruses. These immune mechanisms typically break down the pathogen’s DNA to render it harmless. A new immune system that employs another mechanism for invader neutralization has been identified in the research group of Assistant Professor Daan Swarts from Wageningen University & Research’s Laboratory of Biochemistry. The findings have been published in the scientific journal Cell.
A constant arms race is taking place deep within our bodies. On the one hand, viruses are constantly looking for new methods to enter our cells, while on the other hand, our bodies are constantly developing better defense mechanisms to combat these viruses. This is how sickness and health are normally kept in check. Bacteria and their harmful ‘invaders’, viruses, and plasmids, are engaged in the same arms race.
In an article published in the scientific journal Cell, Ph.D. candidate Bel Koopal from Daan Swarts’ research group proposes a new defense mechanism in this weapons race. The researchers show that after detecting the invading DNA, a novel form of bacterial “Argonaute protein” purposefully breaks down all molecules with the evocative name “nicotinamide adenine dinucleotide (NAD+).
The cell is completely shut down.
Argonaute proteins are found in multicellular creatures such as plants and humans, as well as unicellular animals such as bacteria. These argonautes have been programmed with a tiny strand of “guide RNA” or “guide DNA” to detect invading RNA or DNA with the same sequence. In most situations, the intruder is subsequently split up into smaller, harmless parts and destroyed. Although Swarts’ Argonaute protein employs guide RNA, it protects in a fundamentally different way: after identifying invasive DNA, it fully shuts down the cell by breaking down NAD+.
Bacteria that have advanced
This immune system has been discovered in various bacterial species. Swarts was unsurprised that these unicellular organisms have such sophisticated defense mechanisms. People frequently underestimate the capabilities of microbes, “he argues.” Bacterial immune systems, no matter how little, have been evolving for millions of years and have gotten increasingly sophisticated. They have to because viruses are typically rather smart as well.
In the future, we might be able to detect diseases in the human body using this kind of genetic tools.
Daan Swarts
Daan Swarts speculates, “In the future, we may be able to detect disorders in the human body using these kinds of genetic technologies.”
Swarts, Koopal, and their colleagues conducted the study primarily to get a scientific understanding of the mechanics of Argonaute proteins. Swarts, on the other hand, feels that these discoveries will have practical uses in the long run. For example, the researchers proved that the immune system can be separated and then reprogrammed with a specific strand of guide RNA. Due to the ease with which NAD+ breakdown can be detected, the Argonaute protein can be utilized to recognize certain DNA sequences on command. Swarts sees a future in which “we could be able to detect disorders in the human body using these kinds of genetic technologies.” But we haven’t arrived yet. For the time being, we are driven by a deep curiosity. “
A brand-new immune system
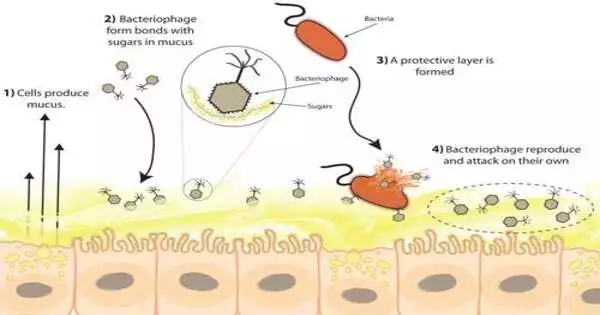
The researchers collected mucus from animals and humans, including a sea anemone, a mouse, and a person, and discovered that bacteriophages cling to the mucus layer on all of them.
They placed bacteriophage on top of a layer of mucus-producing tissue and found that the bacteriophage established bonds with sugars in the mucus, leading to it sticking to the surface. They then exposed these mucus cells to E. coli bacteria and discovered that the bacteriophage attacked and destroyed the E. coli in the mucus, essentially building an anti-microbial barrier on the host that protected it from infection and sickness.
To corroborate their discovery, the researchers did parallel experiments using bacteriophage and E. coli to challenge non-mucus-generating cells. The results showed that samples with no mucus had three times the amount of cell death.
Taking past studies into account, Barr said, “we can suggest the Bacteriophage Adherence to Mucus—or BAM—is a new model of immunity that stresses the vital role bacteriophages play in protecting the host from invading pathogens.”
A shady protector
According to Barr, one of the things that makes this discovery so groundbreaking is that bacteriophages are already prevalent in all humans and animals.
The bacteriophage is recruited from the environment by the body and naturally adheres to mucus layers in many regions of the body, including the mouth and gut. The bacteriophage then becomes its host’s guardian, collecting and attacking its own.
“Not only does this discovery offer a new immune system, but it also indicates the first symbiotic link between phage and animals,” Barr explained. “It will have a huge impact on a variety of disciplines.”
“The study might be applied to any mucosal surface,” Barr explained. “We imagine BAM influencing the prevention and treatment of mucosal infections in the gut and lungs, as well as being used for phage therapy and even directly engaging with the human immune system.”