New exploration gives new insight into how a significant class of atoms are made and moved in human cells.
For quite a long time, researchers knew that mitochondria—specific designs inside cells in the body that are fundamental for breath and energy creation—were engaged in the gathering and development of iron-sulfur cofactors, probably the most fundamental mixtures in the human body. Yet, as of recently, analysts didn’t see how the very cycle functioned.
New exploration, distributed in the journal Nature Communications, observed that these cofactors are moved with the assistance of a substance called glutathione, a cell reinforcement that forestalls specific kinds of cell harm by shipping these fundamental iron cofactors across a film boundary.
Glutathione is particularly valuable as it supports managing metals like iron, which is utilized by red platelets to make hemoglobin, a protein expected to assist with conveying oxygen all through the body, said James Cowan, co-creator of the review and a recognized college teacher emeritus in science and organic chemistry at Ohio State.
“Iron mixtures are basic for the legitimate working of cell chemistry, and their gathering and transport is an intricate cycle,” Cowan said. “We have decided how a particular class of iron cofactors are moved starting with one cell compartment then onto the next by the utilization of perplexing sub-atomic hardware, permitting them to be utilized in various strides of cell science.”
“Iron compounds are necessary for appropriate cellular biochemistry functioning, and their construction and transport is a complex process. We discovered how a certain class of iron cofactors is transported from one cellular compartment to another via complicated molecular machinery, allowing them to be utilised in various processes of cellular chemistry.”
James Cowan, a distinguished university professor emeritus in chemistry and biochemistry at Ohio State.
Iron-sulfur groups are a significant class of mixtures that do various metabolic cycles, such as assisting with moving electrons in the creation of energy and making key metabolites in the cell, as well as aiding the replication of our hereditary data.
Yet, when these groups don’t work as expected, or when key proteins can’t get them, then awful things occur, “Cowan said.
If unfit to work, the ruined protein can lead to a few illnesses, including uncommon types of pallor, Friedreich’s ataxia (an issue that causes moderate sensory system harm), and a huge number of other metabolic and neurological problems.
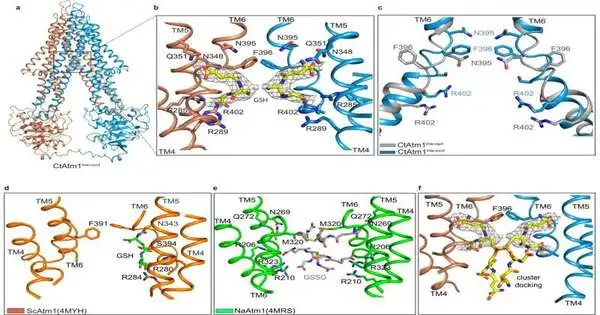
So to concentrate on how this fundamental system functions, scientists started by taking a growth called C. thermophilum, recognizing the key protein atom of interest, and creating huge amounts of that protein for primary assurance. The review noticed that the protein they concentrated on inside C. thermophilum is basically a phone twin of the human protein ABCB7, which moves iron-sulfur groups in individuals, making it the ideal example to concentrate on iron-sulfur group trade in individuals.
By utilizing a mix of cryo-electron microscopy and computational display, the group was then ready to make a progression of primary models itemizing the pathway that mitochondria use to trade iron cofactors to various areas inside the body. While their discoveries are crucial to studying the essential structural blocks of cell natural chemistry, Cowan said he’s eager to perceive how their disclosure could later propel medication and therapeutics.
“By understanding how these cofactors are gathered and moved in human cells, we can lay the basis for deciding how to forestall or ease side effects of specific illnesses,” he said. “We can likewise involve that key information as the basis for different advances in grasping cell science.”
More information: Ping Li et al, Structures of Atm1 provide insight into [2Fe-2S] cluster export from mitochondria, Nature Communications (2022). DOI: 10.1038/s41467-022-32006-8
Journal information: Nature Communications