Biologists are investigating how engineered biofilms closely resemble natural ones. Their findings could aid in the development of drugs to combat the negative effects of these microorganisms that adhere to surfaces.
Bacteria in natural and engineered environments frequently exist as multicellular aggregates embedded in a self-produced matrix of extracellular polymeric substances (EPS), known as biofilms. Biofilms are central to several grand challenges that we must address in the twenty-first century, such as clean water access, and have a significant economic impact on industries ranging from environmental, agricultural, chemical, medical, energy, and manufacturing. The term ‘biofilm engineering’ was coined in the early 1990s by Montana State University’s Center for Biofilm Engineering, where it broadly referred to fundamental and applied biofilm research motivated by industrial, environmental, and health concerns.
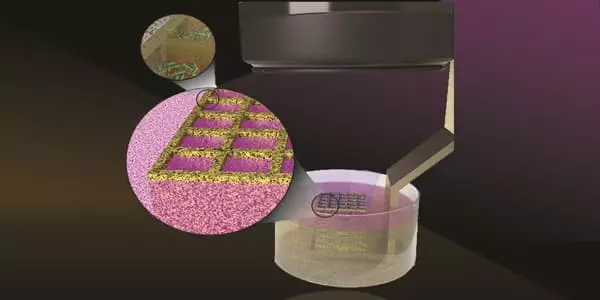
Anne S. Meyer, an associate professor of biology at the University of Rochester, and her colleagues at the Delft University of Technology in the Netherlands recently developed a 3D printing technique for engineering and studying biofilms, which are three-dimensional communities of microorganisms such as bacteria that adhere to surfaces. The findings are important for developing synthetic materials and drugs to combat the negative effects of biofilms.
This paper demonstrates that our engineered biofilms can behave like native biofilms in many ways, including exhibiting emergent drug resistance, making them good model systems for anti-biofilm drug development.
Anne S. Meyer
Biofilms can be both harmful and beneficial to humans: they can coat the surfaces of materials and objects, including medical devices, causing infections and are resistant to many drugs and disinfectants. Biofilms, on the other hand, have the ability to degrade toxic chemicals and environmental pollutants, making them useful in areas such as wastewater treatment.
Meyer and her colleagues demonstrate that engineered biofilms can behave similarly to natural ones in their latest study, which was published in the journal ACS Synthetic Biology. The researchers developed a 3D printing technique that allows them to synthetically engineer and study biofilms made of Escherichia coli (E. coli) bacteria. The technique will allow researchers to better study the properties of biofilms so they can harness their beneficial aspects and combat their harmful effects.
Beneficial biofilms are mostly studied in the context of environmental biotechnology for water purification and resource recovery. The heterogeneity of structure and activity, as well as the lack of control over biofilm dynamics, limit the applications of beneficial biofilms. Effective control of biofilm structure and dynamics would enable and/or facilitate many biotechnological applications, for example, efficient and controllable biofilm-mediated biocatalysis for chemical production.
Microbial fuel cells (MFCs) are emerging as a promising future technology for a wide range of applications, including renewable energy generation. Microorganism-produced electroactive (EA) biofilms are key players in bio-electrochemical systems involving microorganism-mediated electrocatalytic reactions. As a result, genetically modifying the organism to increase EA biofilm production and improve the extra electron transfer (EET) mechanisms may contribute to an increase in MFC current density and COD removal in wastewater treatment plant coupled MFC systems.
The organisms’ extracellular polysaccharides (EPS) contribute to both biofilm formation and electron transfer. Although cell surface modification, media optimization, and validation of operation parameters are well-established strategies for improving fuel cell performance, engineering the critical genes involved in electroactive biofilm formation is the future hope.
“This paper demonstrates that our engineered biofilms can behave like native biofilms in many ways, including exhibiting emergent drug resistance,” Meyer says, “making them good model systems for anti-biofilm drug development.”
Meyer’s lab has been leading a series of research efforts to develop synthetic materials that mimic nature. The materials are used in a variety of industries, including energy, medicine, technology, and fashion. The Meyer group used bacteria to create artificial nacre and graphene, as well as other 3D printing techniques, such as a novel bio-printing technique for printing algae into living, photosynthetic materials.