By using an improved mechanochemical method, the formerly time-consuming but frequently used Birch reduction can now be completed in the air in under a minute.
The Birch reduction is a reaction that is frequently used to produce drugs and bioactive compounds, but the strenuous procedure frequently necessitates that chemists handle liquid ammonia, use cryogenic temperatures, and complete time-consuming steps.
A streamlined technique for carrying out the Birch reduction has been created by researchers at the Institute for Chemical Reaction Design and Discovery (WPI-ICReDD) in Hokkaido University. This technique can be used at room temperature or in ambient air and is 20–150 times faster than traditional approaches. Angewandte Chemie International Edition publishes their findings.
“In previous research, we discovered that using a ball mill for reactions of metals such as magnesium and calcium with organic compounds improved the reaction rate and greatly simplified the process,”
Associate Professor Koji Kubota.
Although several lithium-based techniques for the Birch reduction in solution have been developed, these techniques still required intricate reaction setups with an inert atmosphere or dehydrated conditions because lithium reacts with both air and water. Researchers in this study recognized a chance to avoid these problems by switching from a solution-based method to a solvent-less method using a ball mill, in which reactants are rapidly shaken in a small metal jar along with a metal ball that smashes the solid reactants together.
According to co-author Associate Professor Koji Kubota, “in earlier studies, we found that using a ball mill for reactions of metals like magnesium and calcium with organic compounds improved the reaction rate and greatly simplified the process.”. “In light of this, we considered whether we might be able to create a simpler Birch reduction process by performing reactions between lithium metal and aromatic compounds in a ball mill.”.
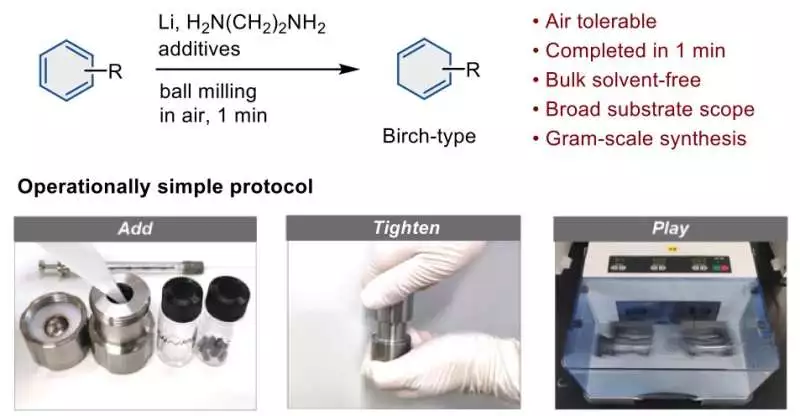
An overview of the streamlined procedure for the Birch reduction using a ball mill. Credit: Angewandte Chemie International Edition (2023). DOI: 10.1002/anie.202217723
The key to this method is that the mechanical force of the ball smashes through the air-reactive surface layer on the lithium, exposing the pure lithium beneath to the other reactants and allowing the Birch reduction to proceed. This method is much simpler to use because it can be done in ambient air and at room temperature.
Researchers proved the process’ adaptability by successfully testing it on a wide range of organic compounds, including pharmaceutical intermediates and other bioactive molecules. The birch reduction was typically finished in an astoundingly short amount of time—one minute.
The team believes that this method could enable the simplified synthesis of a wide range of molecules and represent a significant advancement in mechanochemistry. The procedure was successfully scaled up to larger gram-scale batches.
The study’s principal investigator, Professor Hajime Ito, said, “The Birch reduction is widely used in drug discovery and various chemical industries, and our research has made significant advances, leading to a much simpler and more environmentally friendly Birch reduction process. We anticipate that this development will hasten the search for new drugs as well as numerous other chemical research fields.”.
More information: Yunpeng Gao et al, Mechanochemical Approach for Air‐Tolerant and Extremely Fast Lithium‐Based Birch Reductions in Minutes, Angewandte Chemie International Edition (2023). DOI: 10.1002/anie.202217723





