Due to their similarity to graphene, two-dimensional (2D) boron sheets known as borophenes have recently piqued the interest of material scientists. Be that as it may, comprehension of how the material can be steady in the 2D structure is as yet missing, chiefly because of boron’s extraordinary electron-lacking nature.
In science, molecules in a steady material by and large submit to the octet rule. A carbon atom typically shares eight electrons with its neighbors through four chemical bonds, specifically 3 and 1 bonds for carbon atoms in graphene. While a boron molecule has just 3 valence electrons, its solidity, or its holding system, to have 8 electrons, has been a drawn-out secret ever since.
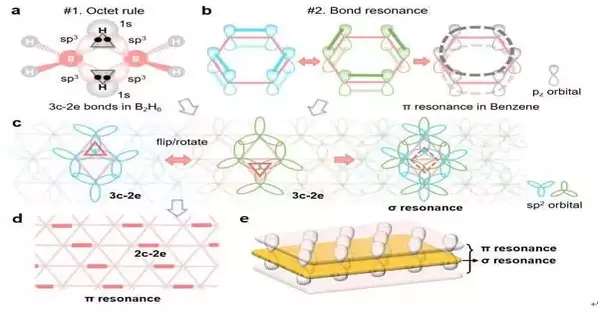
The idea of the three-center, two-electron (3c-2e) bond, which was awarded the 1976 Nobel Prize in Chemistry, helps us comprehend how a boron atom complies with the octet rule in boron-related small molecules like diborane (B2H6). However, it is still unknown how boron atoms in complex boron materials like borophenes adhere to the octet rule and remain stable.
“For the first time, our theory provides fundamental and necessary elements for studying flat boron materials without performing any quantum calculations,”
Author of the study, Prof. Feng Ding,
Bond resonance or aromaticity, which delocalizes electrons out of the plane and into a larger area, could further stabilize carbon materials like benzene. Might we at any point stretch out the hypothesis to the 2D boron sheet to make sense of its three-sided grid-based construction and soundness?
A new bonding theory was proposed by researchers from UNIST’s Departments of Materials Science and Engineering and Mechanical Engineering in collaboration with those from Rice University in the United States and Nankai University in China. This theory explains both how each boron atom in a borophene satisfies the octet rule based on the unique 3c-2e bonds and how the resonance of alternating 3c-2e bonds further stabilizes the 2D sheet in its triangular lattice.
Intriguingly, this theory introduces a novel form of resonance that permits the delocalization of electrons within the 2D plane, analogous to resonance in carbon materials. The triangular boron sheet actually has an out-of-plane resonance and a sandwich electronic structure made up of both in-plane and out-of-plane delocalized electrons.
The bonding structures of these new boron materials can be drawn by anyone, according to the theory, just like the benzene molecule’s Kekulé structures. Hence, the security and properties of the borophene materials can be easily perceived without performing confounding quantum estimations.
For the first time, significant controversies in the field, such as how substrate doping influences the hole concentration in borophene, how hexagonal holes stabilize the triangular boron lattice, and why neutral borophene with a hole ratio of 1/9 is the most energetically advantageous, Dr. Lu Qiu, the study’s first author, said, “The theory reveals the origin of the unique properties of these flat boron materials and therefore offers an avenue for the controlled synthesis and design of borophene by predicting their stabilities on the substrates.”
Natural comprehension of holding in different atoms and materials is consistently the center piece of science.” “We are confident that this bond resonance theory will further stimulate the community towards accelerating the design and synthesis of boron-related materials, like the aromaticity theory for carbon materials,” says Prof. Feng Ding, the study’s corresponding author. “Our theory, for the first time, provides fundamental and necessary elements for studying flat boron materials without performing any quantum calculations.”
Nature Communications is the journal that published the work.
More information: Lu Qiu et al, Theory of sigma bond resonance in flat boron materials, Nature Communications (2023). DOI: 10.1038/s41467-023-37442-8