A new experiment at the world’s largest particle physics laboratory demonstrates how the gas-phase form of iodine, known as iodic acid, forms and suggests it plays a catalytic role in the formation of atmospheric particles.
An international team led by researchers from the University of Colorado Boulder has cracked the chemical code that drives the formation of iodine particles in the atmosphere, revealing how the element contributes to increased cloud cover while depleting molecules in the Earth’s protective ozone layer.
The study, which was carried out at the European Organization for Nuclear Research (CERN), the world’s largest particle physics laboratory, was published today in the journalNature Chemistry. It is the first time that any experiment in the world has demonstrated the mechanism for how the gas-phase form of iodine, known as iodic acid, forms, and it suggests that it plays a catalytic role in the formation of atmospheric particles.
It comes at a time when atmospheric iodine levels are increasing globally, with current levels being three times higher than they were 70 years ago. Researchers hope that this new understanding of iodine’s atmospheric interactions can be added to global atmospheric and climate models to help scientists better understand its environmental impacts, such as increased cloud cover, which could exacerbate the thinning of Arctic sea ice caused by global warming.
This is an excellent example of experiments and computations working together to answer a question that neither could have answered on their own.
Theo Kurten
“This paper establishes a link between the sources of iodine, how they are emitted into the atmosphere, and particle formation, which through subsequent growth, seeds clouds,” saidRainer Volkamer, co-lead author on the paper, professor of chemistry at CU Boulder and fellow at the Cooperative Institute for Research in Environmental Sciences (CIRES). “That link didn’t exist before, and now we have established that link at the molecular level.”
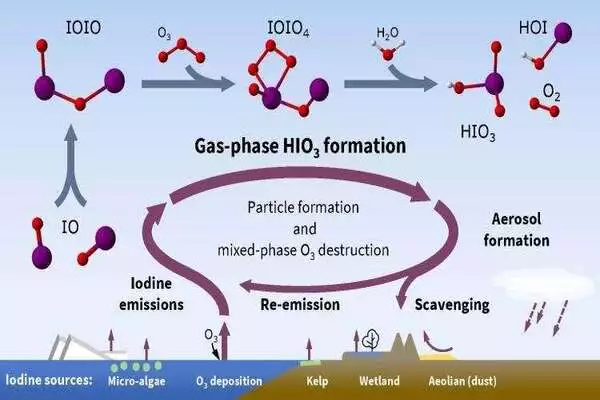
This link between iodine sources and atmospheric particle formation is a multi-step process. First iodine oxide radicals bond with themselves, then react with ozone and water to make iodic acid, with (singlet) oxygen and hypoiodous acid as co-products.
Iodine is a common and highly reactive element that forms radical species in the atmosphere that undergo rapid chemical reactions lasting seconds to minutes. The majority of the iodine in the atmosphere comes from the ocean, where it exists as iodide, which is also found in table salt. Its tripling in the atmosphere over the last 70 years has been linked to an increase in anthropogenic air pollution: when harmful ground-level ozone reacts with ocean-based iodide, it emits volatile iodine gasses into the atmosphere.
While iodine has been studied for 150 years, it has only been in the last two decades that researchers like Volkamer have discovered its critical role in the atmosphere. In 2020, Volkamer and CU Boulder and CIRES researchers published research showing how iodine reaches the stratosphere and eats away at the ozone that protects the planet from harmful UV radiation.
“Iodine is the new kid on the block, among other halogens, that play into the recovery of the ozone layer,” said Volkamer.
DisCERNing chemical processes
To investigate this missing link, the researchers turned to CERN, which has the ideal conditions for observing and collecting data on these particles. Here, the CLOUD (Cosmics Leaving Outdoor Droplets) experiment has become the world’s leading laboratory experiment for studying the remaining unknown aspects of aerosol and cloud formation.
Volkamer’s research group, the Atmospheric Trace Molecule Spectroscopy (ATMOSpec) Lab, is one of only three universities in the United States (along with Caltech and Carnegie Mellon University), as well as 16 European partners, to be a part of this collaboration.
“This is the only such experiment that exists in the world,” said Volkamer. “It’s an honor to be part of the collaboration and to be leading it in the context of a study like this one.”
In the CLOUD chamber at CERN, the researchers had access to a laboratory environment with perfect control over conditions like temperature, pressure, humidity, ozone concentration, and iodine concentration, as well as access to different light sources resembling different aspects of the solar spectrum. By setting up this artificial, indoor atmosphere where certain reactions may or may not happen, scientists could accurately gather data on iodine chemical reactions that form and grow particles.
“This is an excellent example of experiments and computations working together to answer a question that neither could have answered on their own,” said Theo Kurten, co-lead author on the study and a professor of chemistry at the University of Helsinki.
They tested their findings in the air surrounding the Mado observatory on Réunion Island in the southern Indian Ocean, a remote location free of much of the influence of human activity, to see if what they saw in the lab translated to the real world, and were able to corroborate their laboratory results.
A catalytic role on climate
Unlike other elements such as sulfur (or sulfuric acid), iodine doesn’t need the help of other molecules (known as “bases”) to form atmospheric particles. It’s also quite efficient at this process, compared to other elements, the researchers found. Therefore, the formation of particles from iodic acid is not limited to coastal iodine hot spots, or locations where these chemical bases are available, but rather can occur throughout the atmosphere.
“It’s a global phenomenon, and the global significance of iodine in particle formation may be greater than previously thought,” said Henning Finkenzeller, the study’s first author and part of his dissertation at CU Boulder.
According to Finkenzeller, iodine is fundamentally different from other particle-forming vapors because of its ability to kick-start the formation of atmospheric particles and the fact that a single iodine atom can kick-start this process multiple times. This catalytic role in particle formation enhances its effects in the atmosphere, whether that role is to eliminate protective ozone molecules or to increase cloud cover.
Because of our negative effects on global air quality, human activity increases the availability of iodine in the atmosphere, and the effects of this short-lived element may be long-lasting. As Arctic sea ice melts, more iodine enters the atmosphere, increasing cloud cover and amplifying the region’s warming effects. Storms in the tropics can propel this iodine high into the atmosphere, where it interacts with our protective ozone layer.
“We still need to learn more about the chemistry of iodine recycling. But, now that we know how excess iodine affects particle formation, clouds, and ozone recovery in our planet’s atmosphere, we are one step closer to understanding how it works” Volkamer stated.





