One of the most significant chemical reactions in industry is the Haber-Bosch (HB) process. It combines nitrogen and hydrogen gases with an iron-based catalyst under extreme heat and pressure to create ammonia fertilizer, which aids in feeding over five billion people.
In an effort to increase ammonia yield while using less energy, researchers have attempted to lower the reaction temperature of the HB process over the years. They recently created new catalysts based on transition metals like ruthenium, cobalt, and nickel, which have much higher catalytic activity than iron, in order to achieve this.
However, at low temperatures of 100 to 150 oC, these catalysts preferentially adsorb hydrogen atoms onto their surfaces, which inhibits the production of ammonia by reducing nitrogen adsorption. The hydrogen poisoning phenomenon is a barrier to the low-temperature HB process.
“Iron-based catalysts are not susceptible to hydrogen poisoning. As a result, they can be employed in the low-temperature HB process, but only when paired with an appropriate promoter that boosts their catalytic activity.”
Professor Michikazu Hara of the Laboratory for Materials and Structures at Tokyo Institute of Technology (Tokyo Tech).
In light of this, scientists working under the direction of Professor Michikazu Hara of the Laboratory for Materials and Structures at the Tokyo Institute of Technology (Tokyo Tech) have turned their attention back to iron-based catalysts and modified them to produce ammonia at 100 °C. The American Chemical Society Journal is prepared to publish their work.
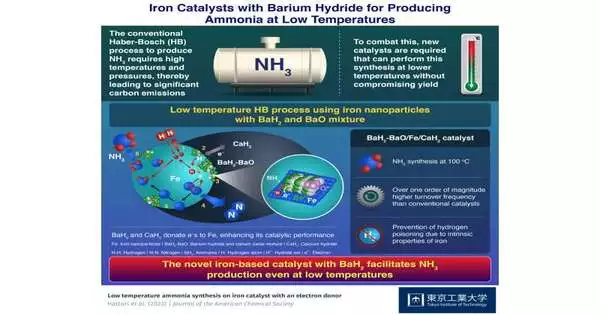
Prof. The purpose of the research is explained by Hara. For iron-based catalysts, hydrogen poisoning is not a serious issue. They can therefore be used for the low-temperature HB process, but only in conjunction with a suitable promoter that raises their catalytic activity. In this research, barium oxide (BaO) and barium hydride (BaH2) were combined and deposited on calcium hydride (CaH2) particles to create metallic iron (Fe) nanoparticles.
A series of experiments showed that both hydrides’ hydride ions and iron nanoparticles have strong interactions with one another. Consequently, hydrogen atoms transfer from hydrides to nanoparticles and become desorbed as hydrogen gas, leaving electrons behind. These electrons are provided to the iron nanoparticles by the hydrides. It makes it easier for nitrogen gas to split into atoms, which improves the catalytic activity for ammonia production even at low temperatures.
Under a moderate pressure of 0 9 MPa, the BaH2-BaO/Fe/CaH2 catalyst demonstrated a turnover frequency of 0 23 s-1 at 100 °C and 12 3 s-1 at 300 °C. These numbers, which are several orders of magnitude higher than those for catalysts made of other transition metals, are due to iron’s capacity to stop hydrogen toxicity by desorbing hydrogen atoms from adsorbed materials as hydrogen gas at low temperatures.
Speaking about the potential for their work in the future, Prof. The low-temperature HB process, which uses less energy, is made possible by the BaH2-BaO/Fe/CaH2 catalyst, according to Hara. As a result, it may help to cut down on the use of fossil fuels and, consequently, the global carbon emissions. Iron is also widely available and reasonably priced, which increases the sustainability of the HB process.”.
More information: Michikazu Hara et al, Low temperature ammonia synthesis on iron catalyst with an electron donor, Journal of the American Chemical Society (2023). DOI: 10.1021/jacs.2c13015