Another focus by UT Southwestern analysts proposes that fat cells, or adipocytes, that fill in nearness to bosom diseases, can move into other cell types that advance growth development. The findings, published in Cell Reports, could lead to better approaches to combating breast cancer, a disease that affects over 300,000 women in the United States each year and kills nearly 45,000 people.
“We recognized novel adipocyte-determined cell types in the mammary organ that offer a ripe soil for bosom disease cancer attack and development,” said concentrate on pioneer Philipp Scherer, Ph.D., teacher of inner medication and cell science and an individual from the Harold C. Simmons Thorough Disease Place at UTSW.
Weight has for some time been viewed as a risk factor for bosom disease events and more terrible guesses. Studies have shown that fat cells in close contact with bosom cancer cells have an upgraded capacity to separate their lipids to give fuel to attacking growth cells. In any case, as Dr. Scherer made sense of, it has been hazy what different jobs these adipocytes play in the bosom disease movement.
“We discovered novel adipocyte-derived cell types in the mammary gland that provide a suitable soil for breast cancer tumor invasion and proliferation.”
leader Philipp Scherer, Ph.D., Professor of Internal Medicine and Cell Biology
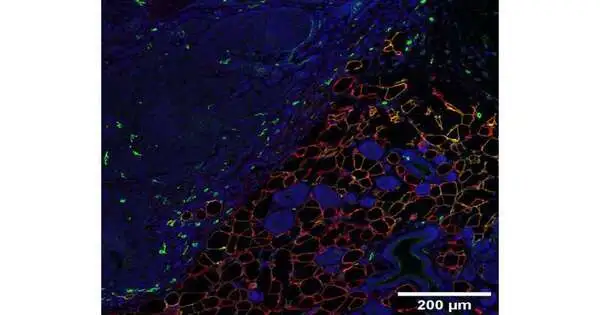
To respond to this inquiry, Qingzhang Zhu, Ph.D., a Teacher of Inner Medication and an individual from the Scherer lab, and his partners utilized a hereditary method that “painted” adipocytes in lab mice so they shone a fluorescent tone, making it conceivable to follow these cells over the long haul.
When the analysts embedded bosom cancers in the mice or hereditarily controlled the rodents’ own bosom cells to transform them into growth cells, they saw that close by fat cells shrank and took on structures not the same as local adipocytes. Hereditary testing to recognize which qualities were dynamic in these fat cells showed that these cells previously relapsed to a prior stage of being developed, then slowly evolved hereditary markers of other cell types, including connective tissue cells, muscle cells, and safe cells.
Further examination showed these changed fat cells urged bosom disease cancers to develop. However, this property was also based primarily on their ability to supply energy to neighboring growth cells.Also, the properties of the cell types that fat cells transform into after they lose their lipids and their fat cell character are significant since they add altogether to the nearby fibrosis, which adds to the firmness of bosom tissue. When the analysts improved the lipid-putting away limit of mature fat cells, they failed to transform into other cell types and have not yet advanced cancer development.
Dr. Scherer said the system for how adipocytes change into other cell types isn’t yet clear; in any case, a compound signal from growth cells is likely liable for this peculiarity. He and his partners intend to look for this sign and search for alternate ways of controlling this framework to put bosom disease development down.
More information: Qingzhang Zhu et al, Adipocyte mesenchymal transition contributes to mammary tumor progression, Cell Reports (2022). DOI: 10.1016/j.celrep.2022.111362
Journal information: Cell Reports