What number of battery-powered lithium-particle batteries would you say you are wearing? How many are in your general area?
They’re probably more than a couple, and they’re ideal for powering everything essential to modern life: cellphones, watches, PCs, vehicles, and so on.
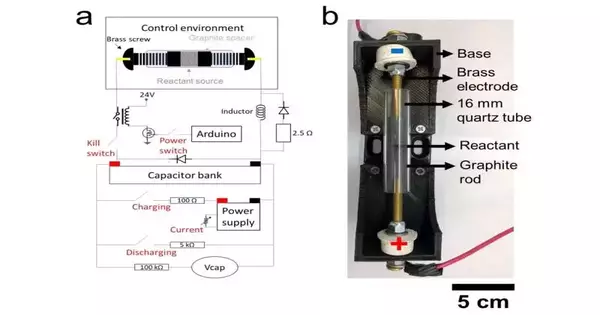
Yet, where they go when they fizzle is a developing issue. Rice College researchers accept they have a halfway arrangement that depends on the novel “streak” Joule warming cycle they created to deliver graphene from squander.
The Rice lab of physicist James Visit has reconfigured the cycle to rapidly recover graphite anode materials found in lithium-particle batteries, eliminating pollution so they can be utilized over and over.
“By 2026, lithium-ion battery manufacturing is predicted to be five times what it was in 2017, and currently, less than 5% of them are recycled. This places a huge burden on the environment, as these worn batteries are processed and the anodes are burned for energy or sent to landfills.”
The Rice lab of chemist James Tour
The lab’s work shows up in Cutting Edge Materials.
Blazing powdered anodes from business batteries reuse some of the “faltering” waste accumulation they are currently abandoning, according to analysts.In only a couple of moments, a shock of high energy decays inorganic salts, including lithium, cobalt, nickel, and manganese, from an anode. These can be recovered by handling them with weak hydrochloric acid.
“The creation of lithium-particle batteries in 2026 is supposed to be multiple times what it was in 2017, and at this moment, under 5% of them are reused,” said Visit, who presented the blaze cycle for graphene in 2020. “That puts a weighty burden on the climate as these spent batteries are handled and the anodes are consumed for energy or sent to landfills.”
“We’re guaranteeing our cycle can recuperate basic metals and recondition anodes in an undeniably more earthy and monetarily cordial way,” he continued.
The study found that hot anodes degrade the strong electrolyte interphase (SEI), which conducts lithium particles while also protecting the anode from adverse reactions.
Blazing then covers the excess graphite particles with a particle-sized porous carbon shell that adds to their future limit, rate execution, and cycling security, contrasted with materials routinely reused in a tedious and energy-intensive cycle known as high-temperature calcination.
The lab assessed that it would cost about $118 to reuse one ton of untreated anode squander. They demonstrated that streak reused anodes have a recovered explicit limit of 351 milliamp hours per gram at 32 degrees Fahrenheit, which is superior to the rate execution and electrochemical security of untreated or calcined reused anodes.
The reused, streaked anodes the analysts tried held over 77% of their ability after 400 re-energizing cycles.
“Past the spent graphite anodes, we are sure that the cathodes, the electrolytes, and their blends can really be reused or reconditioned by our strategy,” said Rice graduate understudy Weiyin Chen, the lead creator of the review.
More information: Weiyin Chen et al, Flash recycling of graphite anodes, Advanced Materials (2022). DOI: 10.1002/adma.202207303
Journal information: Advanced Materials