In “The Princess and the Pea,” a fairy tale by Hans Christian Andersen published in 1835, the princess is praised for her sensitivity to a pea positioned under 20 mattresses on her bed. A new study from the McKelvey School of Engineering at Washington University in St. Louis has discovered that unlike normal cells, which are unable to sense a layer of cells beneath the top collagen layer on which they normally travel, cancer cells are able to sense this layer, much like the fabled princess.
Amit Pathak, an associate professor of mechanical engineering and materials science, and Christopher Walter, a postdoctoral research associate in Pathak’s lab, discovered that cancer cells have what they term “depth mechanosensing,” or the capacity to sense the characteristics of distant environments beneath their immediate extracellular matrix. This may imply that extracellular matrices that are not inside of cells can control cell migration through their mechanical characteristics. On April 5, Cell Reports published the findings of their study online.
For many years, Pathak and his group have investigated how cells move in order to understand how cancerous tumors invade tissue, how wounds mend, and how organs and tissues grow. They have previously demonstrated that cells move more quickly in stiffer environments than in soft ones due to higher forces and tractions.
“Cells sense deeply into their environment, and their polarity installs a polarity in the extracellular matrix based on what the cell tells it,”
Amit Pathak, an associate professor of mechanical engineering and materials science
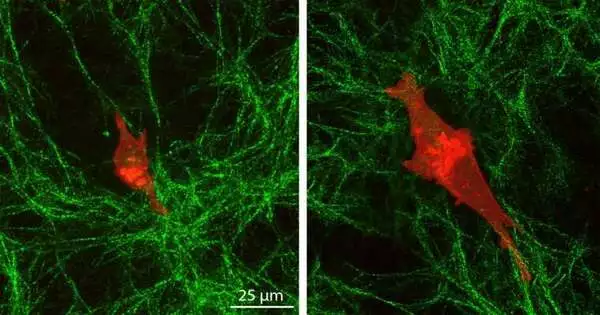
In their recent work, Pathak and Walter made two hydrogels—one stiff and one soft—and then covered them with an extracellular matrix made of collagen. They observed the behavior of triple-negative breast cancer cells and normal human mammary epithelial cells on the surfaces using a wide range of techniques, including atomic force microscopy, high-resolution imaging, and analyses of cell migration dynamics.
Normal cells moved slowly on top of the collagen layer and exhibited little change in size or shape. Due to their depth mechanosensing, cancer cells on the rigid underlying gel lengthened and extended more stable protrusions that aid in their communication across matrix layers, migrated more quickly, and produced greater pulling of collagen fibers.
According to what the cell is telling the extracellular matrix, “cells sense deeply into their environment, and their polarity installs a polarity in the extracellular matrix.”. These behaviors indicate that, in contrast to normal cells, which only react to the immediate, soft collagen gel, cancer cells are shaped by the stiffness of the underlying gel. There was no prior knowledge of this.
According to Walter, the polarity of the cells also aids in the generation of force for the cells to move in a specific direction, but they also require the capacity to pull on collagen fibers and alter their environments to make them more conducive to migration.
Walter explained, “By cutting the collagen itself or cross-linking it, we were able to get rid of their ability to sense the distant matrix, eliminating the feedback that the matrix gives the cells.” The feedback stops if either mechanism is lost, so they must have both.
According to Pathak, the cell needs this feedback from the surface. In order to explain the deep sensing seen in experiments, Jairaj Mathur, a doctoral student in mechanical engineering and materials science, created a computational model to integrate the polarized forces of cells with collagen remodeling.
In the absence of this feedback between the cells and the matrix, depth sensing may occur briefly but won’t last, according to his model. “And for this reason, cancer cells excel at this because they can entice the matrix to migrate and sense depth.”.
More information: Christopher Walter et al, Reciprocal intra- and extra-cellular polarity enables deep mechanosensing through layered matrices, Cell Reports (2023). DOI: 10.1016/j.celrep.2023.112362