A global group of scientists has shown that sun-based controlled blend gas could reuse carbon dioxide into fills and helpful synthetics.
“In the event that we can create syngas from carbon dioxide using just sun-based energy, we can use this as an antecedent for methanol and different synthetics and fills. “This will altogether lessen CO2 outflows,” said Zetian Mi, teacher of electrical and PC design at the University of Michigan, who led the review distributed in the Proceedings of the National Academy of Science.
Made mostly out of hydrogen and carbon monoxide with a touch of methane, syngas is usually gotten from petroleum products with the assistance of power. Also, harmful synthetics are frequently added to the cycle to make it more effective.
“Our new approach is actually quite basic, but it’s exciting since it’s non-toxic, sustainable, and incredibly cost effective,” says the researcher.
Roksana Rashid
“Our new cycle is basic, yet it’s energizing since it’s not harmful, it’s feasible, and it’s extremely savvy,” said Roksana Rashid, the first creator of the review, who played out the tests as a doctoral understudy in electrical and PC design at McGill University in Canada.
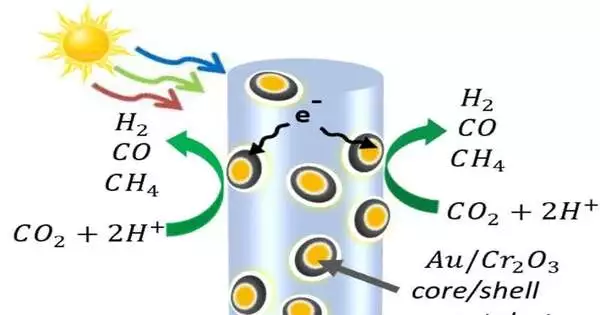
To make a cycle that utilizes just sun-based energy, Mi’s gathering defeated the trouble of parting carbon dioxide particles, which are among the most steady in the universe. For this, they peppered a wood of semiconductor nanowires with nanoparticles. Those nanoparticles, made of gold covered with chromium oxide, pulled in the carbon dioxide atoms and bowed them, debilitating the connections between carbon and oxygen.
The gallium nitride nanowires utilize light energy to free electrons and the decidedly charged spaces they abandon, known as openings. The openings split water atoms, isolating the protons (hydrogen) from the oxygen. Then, at the metal impetuses, the electrons split the carbon dioxide into carbon monoxide and, at times, attract the free hydrogen to make methane. Processes are being worked on to isolate oxygen from different gases.
Mi’s lab at McGill University and U-M. “Our innovation reveals insight into how to fabricate dispersed syngas creation from air, water, and daylight,” said Baowen Zhou, co-creator of the review with Mi and a previous postdoctoral examination individual in Mi’s lab at McGill University and U-M.
By changing the proportion of gold to chromium oxide in the nanoparticles, Mi’s group had the option to control the overall measures of hydrogen and carbon monoxide created in the response. This is significant on the grounds that the proportion of hydrogen to carbon monoxide influences whether creating a kind of fuel or chemical is so natural.
“What is amazing is the collaboration between gold and chromium oxide to make the CO2 decrease to syngas effective and tunable.” “That was impractical with a solitary metal impetus,” Mi said. “This opens up many energizing open doors that were not recently thought of.”
Mi’s tunable syngas arrangement utilizes standard modern assembling processes and is adaptable. While Rashid involved refined water in this trial, seawater and other electrolyte arrangements are likewise expected to work, and Mi has involved them in related water-dividing studies.
“The semiconductor we use as the light safeguard depends on silicon and gallium nitride, which are the most commonly created semiconductors, and we utilize almost no material for the gallium nitride.” “Each nanowire is around one micrometer in thickness,” Mi said.
Mi’s next objective is to build the proficiency of the gadget, which presently remains at 0.89%. When 10% of the light energy is changed over completely to compound energy, he trusts that the innovation could see the innovation be taken on for sustainable power, like sun-based cells.
More information: Roksana Tonny Rashid et al, Tunable green syngas generation from CO2 and H2O with sunlight as the only energy input, Proceedings of the National Academy of Sciences (2022). DOI: 10.1073/pnas.2121174119