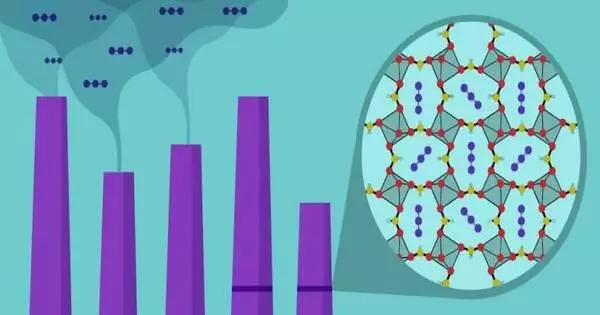
Ceramics that can “breathe” oxygen at lower temperatures can be used to help purify air and remove pollutants. These ceramics, known as oxygen-permeable ceramics, can selectively allow oxygen ions to pass through while blocking other gases. This ability can be used in devices such as oxygen concentrators, which are used to remove nitrogen and other impurities from the air, and produce high-purity oxygen.
Additionally, these ceramics can also be used in catalytic converters to remove pollutants from exhaust gases in automobiles. The oxygen ions from the ceramics can help to oxidize pollutants such as nitrogen oxides (NOx) and hydrocarbons (HC), reducing their harmful effects on the environment and human health. However, it is important to note that this technology is still in the development stage and it is yet not commercially available.
With the shift to electric cars a cumbersome process, improvements to exhaust gas purification in petrol or diesel cars are crucial in the fight to reduce emissions. A research group has developed a Cerium-Zirconium-based oxide that boosts the purifying qualities of ceramics inside catalytic converters – a device attached to conventional cars that convert harmful gases to less-toxic pollutants.
Ceramics with an oxygen storage capacity (OSC) are extremely important in the purification process. They aid in the removal of noxious gases and prevent the precious metals in catalytic converters from coarsening, which reduces the purification capabilities of the converters.
Although electric vehicles (EV) are the focus of much of the discussion about reducing vehicle emissions, their sales remain low, accounting for only 1% of car purchases in Japan in 2021. Meanwhile, the European Union is expected to adopt stricter emission regulations in the near future. As a result, improving the performance and functionality of exhaust gas purification catalysts in gasoline or diesel-powered vehicles is critical in the push toward carbon neutrality.
Almost all gasoline and diesel vehicles are equipped with catalytic converters, which convert harmful hydrocarbons, carbon monoxide, and nitrogen oxide into safer gases such as nitrogen, carbon dioxide, and water vapor. Toxic gases pass through a honeycomb structure that is coated with exhaust gas-purifying catalysts.

Ceramics with an oxygen storage capacity (OSC) are extremely important in the purification process. They aid in the removal of noxious gases and prevent the precious metals in catalytic converters from coarsening, which reduces the purification capabilities of the converters.
However, a lower operating temperature is required to maximize their potential. However, scientists have struggled to achieve this because lowering the temperature below 500 oC results in slower ion diffusion.
Now, a research group at Tohoku University’s Graduate School of Engineering has developed a Cerium-Zirconium-based (Ce-Zr) oxide with excellent OSC at 400 ºC by controlling its crystal structure. The OSC at 400 ºC was higher than conventional materials by a factor of 13.5, even without precious metal catalysts. The key to our success was introducing a tiny amount of transition metals, such as iron, to the Ce-Zr-based oxides,” said Professor Hitoshi Takamura, leader of the research group.
In the oxides, the ‘transition metal doping’ had two distinct effects. It promoted cation ordering and accelerated oxygen diffusion by easing the formation of oxygen vacancies.
“Cation ordering cleans up the crystal structure and makes oxygen more easily released,” Takamura explained. The iron doping reduced the cation-ordering temperature, allowing the Ce-Zr-based oxides to have a larger surface area. This increased their endurance and ability to purify toxic gases. Takamura and his colleagues hope to test the material in the future by loading it with palladium on honeycomb supports.