Hydrogen is used as a fuel in an electrochemical process that combines hydrogen and oxygen to produce electrical energy and water. The reverse electrolysis process, which produces ‘green’ hydrogen and oxygen from water, can use a variety of renewable energy resources (wind, wave, and solar) to produce hydrogen as a fuel for renewable power generation. There is also growing interest in hydrogen power as a uniquely clean energy source that can generate heat and emits only water as a byproduct.
Imperial researchers have created a hydrogen fuel cell that uses iron instead of the rare and expensive platinum, allowing the technology to be used more widely. Hydrogen fuel cells convert hydrogen to electricity with only water vapour as a byproduct, making them an appealing green option for portable power, particularly for vehicles.
However, the cost of one of the primary components has hampered their widespread use. The fuel cells rely on a platinum catalyst, which is expensive and scarce, to facilitate the reaction that generates electricity.
Our cheaper catalyst design should make this a reality, and allow deployment of significantly more renewable energy systems that use hydrogen as fuel, ultimately reducing greenhouse gas emissions and putting the world on a path to net-zero emissions.
Professor Anthony Kucernak
Now, a European team led by Imperial College London researchers has developed a catalyst using only iron, carbon, and nitrogen – materials that are inexpensive and readily available – and demonstrated that it can be used to power a high-power fuel cell. Their findings were published today in the journal Nature Catalysis.
“Currently, around 60% of the cost of a single fuel cell is the platinum for the catalyst,” said lead researcher Professor Anthony Kucernak of Imperial’s Department of Chemistry. To make fuel cells a truly viable alternative to fossil-fuel-powered vehicles, for example, the cost must be reduced.
“Our cheaper catalyst design should make this a reality, and allow deployment of significantly more renewable energy systems that use hydrogen as fuel, ultimately reducing greenhouse gas emissions and putting the world on a path to net-zero emissions.”
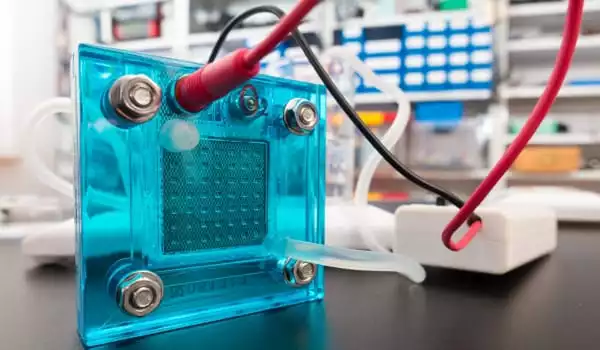
The team’s breakthrough was to create a catalyst in which all of the iron was distributed as single atoms within an electrically conducting carbon matrix. Single-atom iron has different chemical properties than bulk iron, which has all of its atoms clustered together and is therefore more reactive.
Because of these properties, iron boosts the reactions required in the fuel cell, acting as a good substitute for platinum. In lab tests, the team demonstrated that a single-atom iron catalyst performs similarly to platinum-based catalysts in a real fuel cell system.
In addition to producing a cheaper catalyst for fuel cells, the method developed by the team could be adapted for other catalysts for other processes, such as chemical reactions using atmospheric oxygen as a reactant instead of expensive chemical oxidants, and wastewater treatment using air to remove harmful contaminants.
First author Dr Asad Mehmood, from the Department of Chemistry at Imperial, said: “We have developed a new approach to make a range of ‘single atom’ catalysts that offer an opportunity to allow a range of new chemical and electrochemical processes. Specifically, we used a unique synthetic method, called transmetallation, to avoid forming iron clusters during synthesis. This process should be beneficial to other scientists looking to prepare a similar type of catalyst.”
Debates about the benefits and drawbacks of hydrogen fuel cells continue, but despite current limitations, hydrogen is still an environmentally friendly alternative to fossil fuels that can be used to provide flexible and high-density power and propulsion for a wide range of industrial plants and modes of transportation using hydrogen fuel cell technology.
The team worked with Johnson Matthey, a UK fuel cell catalyst manufacturer, to test the catalyst in appropriate systems and hopes to scale it up for use in commercial fuel cells. Meanwhile, they are working to improve the catalyst’s stability so that it can compete with platinum in terms of durability and performance.





