A joint group of scientific experts from Oxford College and IBM Exploration Europe-Zürich has effectively orchestrated a doubly hostile to sweet-smelling C16 carbon allotrope. In their paper distributed in the journal Nature, the gathering portrays their engineered cycle and its conceivable use in investigating new trial hypotheses.
In 2019, an alternate group of physicists blended another allotrope (sub-atomic type) of carbon, C18—it was made in a roundabout structure with the particles connected by exchanging triple and single bonds. The accomplishment proposed suggests that blending a C16-carbon allotrope may be conceivable. Earlier endeavors had bombed because of the intrinsic shakiness of the cycle. In this new exertion, the examination group has conquered issues looked at by others to effectively make a doubly hostile to sweet-smelling C16 carbon allotrope.
To accomplish their accomplishment, the specialists understood that they would have to defeat the unsteadiness of the forerunners since they are hostile to fragrant because of their higher energies. Their methodology included utilizing a few concealing specialists to further develop dependability.
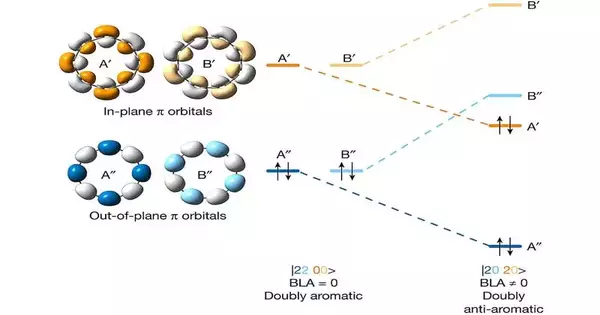
The last union cycle included utilizing a macrocycle that had two bromine and four carbon monoxide substituents, the consequences of which were laid on a substrate of sodium chloride. The group then utilized a burrowing magnifying lens to send picoamperes of charge to explicit locales on the particle. This pushed up the voltage on the bromine to 1.3V and the carbon monoxide to 3V—enough to compel bonds to form between the carbon molecules. The outcome was the development of a polyyne-organized ring kept intact by exchanging triple and single bonds.
The scientists then, at that point, affirmed that they had made the ideal allotrope by zeroing in on their ring with a nuclear power magnifying lens. They likewise observed that they had the option to concentrate on the conveyance of the electrons in the sub-atomic orbitals utilizing a Kelvin test force magnifying lens and the checking burrowing magnifying instrument. This showed that the atom was doubly sweet-smelling, true to form.
The work could pave the way for new examinations and open doors to the investigation of new scientific hypotheses and maybe to the improvement of new materials produced using outlandish carbon allotropes.
More information: Yueze Gao et al, On-surface synthesis of a doubly anti-aromatic carbon allotrope, Nature (2023). DOI: 10.1038/s41586-023-06566-8