Estimating the pH of substances gives us fundamental pieces of information about our general surroundings, for example, distinguishing defiled water or really looking at the poisonousness of clinical or corrective items.
Frequently, just modest quantities of tests are accessible, yet observing the variety in pH in these miniscule volumes matters. For instance, distinguishing pH changes inside minuscule volumes of liquid from single cells can help in the discovery of ovarian malignant growth.
Nonetheless, the ongoing techniques for estimating pH are predominantly for mass arrangements and are not sufficiently delicate or excessively delicate to gauge small volumes on a business scale.
“Our solution had to be eco-friendly, long-lasting, and sensitive enough to accurately measure pH variation in just a few microliters of sample.”
Dr. Qiuchen Dong, who led the study,
In a new report distributed in Microchimica Acta, researchers from Xi’an Jiaotong-Liverpool College, China, have fostered a technique that conquers these issues.
Dr. Qiuchen Dong, who drove the review, says, “Our answer should have been harmless to the ecosystem, solid, and sufficiently delicate to quantify pH variety in only a couple of microliters of tests precisely.”
An absence of choices
A few industrially accessible strategies used to test pH depend on the emotional choices of the natural eye. For instance, utilizing paper strips holding back colors that change tone contingent upon the pH of the substance depends on individuals contrasting the variety against a scale. This results in a critical variety in their responses. Certain individuals might see the variety as pH 7.5, others at 8, for instance. This technique, subsequently, isn’t delicate to a few pH changes, meaning it’s more similar to an estimation. A portion of the colors utilized are likewise harmful to tests, which will influence the pH recorded.
A more touchy technique for estimating pH utilizes very delicate glass cathodes, which are effortlessly broken, so they are normally just utilized in a lab setting.
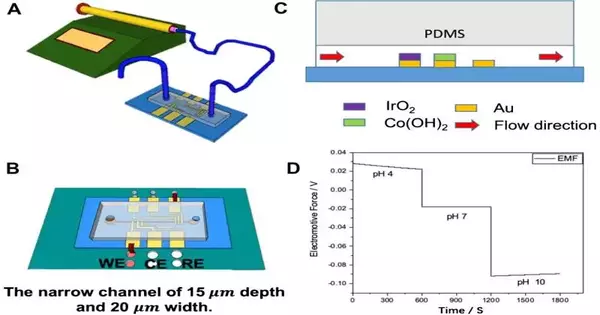
To tackle these issues, Dr. Dong and his postgraduate understudy, Weiyu Xiao, have utilized novel materials and techniques to make a delicate yet vigorous pH sensor.
In Dr. Dong and Xiao’s new pH sensor, liquid examples go through a progression of small channels (microfluidic channels) and north of three profoundly touchy cathodes made from light-responsive layered materials and metals.
“Our answer to the issue depends on creating microfluidic channels and terminals utilizing photolithography, a strategy frequently utilized in the semiconductor fabricating industry.”
These microfluidic pH sensors can identify minor variations in the quantity of protons inside a substance, which characterizes the pH. This permits the estimation of pH with astounding precision.
Future purposes
The group presently has a patent forthcoming for the pH sensor and is creating coordinated efforts with industry designers who will incorporate the innovation into their lab hardware.
“The outcome of this study is down to the difficult work of my ongoing Ph.D. understudy, Weiyu Xiao, who was an expert’s understudy during this work. It is extremely noteworthy to see an understudy arrive at such an undeniable level in such a brief period. She is an extraordinary good example, and I trust different understudies will be enlivened by the amount she has accomplished.
“The work is likewise because of my past associates, Dr. Abdennour Abbas at the College of Minnesota and Dr. Yu Lei from the College of Connecticut, who assisted me with figuring out the thoughts for this undertaking and numerous others.”
The group accepts that their new sensor will have broad business applications, from supporting the identification of malignant growths and vector-borne infections to distinguishing defilement in soil splashed with pesticides.
More information: Weiyu Xiao et al. Iridium oxide and cobalt hydroxide microfluidic-based potentiometric pH sensor, Microchimica Acta (2023). DOI: 10.1007/s00604-023-06035-z