Scientists from the Institute of Solid State Physics, Hefei Institutes of Physical Science of the Chinese Academy of Sciences, revealed a carbon-upheld, Pt-changed MoO3 nanoparticle impetus for electrochemically specific C=O hydrogenation of cinnamaldehyde to create cinnamyl liquor.
Their discoveries were published in Chemical Communications.
Cinnamaldehyde (CAL), a delegate compound for, -unsaturated sweet-smelling aldehydes, has been broadly utilized in hydrogenation responses to yield high-esteem added items, among which cinnamyl liquor (COL) is typically applied as a flavor-added substance and drug natural substance.
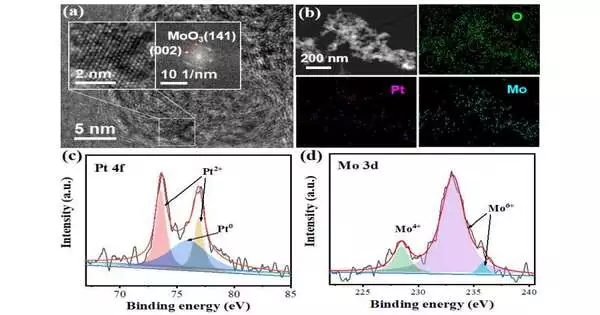
In this work, the analysts orchestrated Pt-altered MoO3 ultrafine particles stacked on dynamic carbon (Pt-MoO3/C). The electrochemical portrayal results showed the clear decrease top after the presentation of CAL, and the solid adsorption of CAL on the terminal surface prompted a fixation expansion in the negative shift of the decrease top.
As an electrocatalyst, Pt-MoO3/C displayed better electrocatalytic hydrogenation movement towards CAL than COL, with a high transformation of almost 100% and selectivity of 78% at -0.4 V, and the Faradaic effectiveness could arrive at half while multiplying the Faraday reciprocals of the electric amount applied.
The estimation results demonstrated that the high selectivity towards COL began from the synergistic impact of the Pt and Mo species, which could specifically adsorb the C=O obligation of the CAL particle by means of vertical adsorption setup, in this way further developing the CAL hydrogenation selectivity to create COL.
More information: Jialu Wang et al, Pt-Modified MoO3 catalyst for the electrochemically selective C=O hydrogenation of cinnamaldehyde, Chemical Communications (2022). DOI: 10.1039/D2CC01527G