Materials with a rigid, cage-like structure are known as metal-organic frameworks (MOFs), and they can be used for a variety of purposes, such as drug delivery and gas storage. The building blocks that make up the materials can be altered, as well as how they are arranged, to create MOFs that are suitable for various applications.
However, not every MOF structure that could exist is reliable enough to be used for tasks like catalyzing reactions or storing gases. MIT researchers have developed a computational method that enables them to predict which MOF structures will be the most stable, which can assist researchers in determining which MOF structures might work best for a specific application.
The researchers have discovered about 10,000 potential MOF structures that they label “ultrastable” using their computational model, making them suitable candidates for uses like converting methane gas to methanol.
“A truly effective MOF material for catalysis or gas storage would have a very open structure, but once you get that open structure, it may be extremely difficult to ensure that the material is also stable under long-term usage,”
Heather Kulik, an MIT associate professor of chemistry and chemical engineering,
According to Heather Kulik, an associate professor of chemistry and chemical engineering at MIT and the study’s senior author, “people don’t necessarily know in advance how stable that material is when they come up with hypothetical MOF materials.”
“We used data and our machine-learning models to generate building blocks that were predicted to have high stability, and when we recombined those in ways that were significantly more diverse, our dataset was enriched with materials with higher stability than any prior set of hypothetical materials people had generated.”.
The paper, which was published today in the journal Matter, is led by Aditya Nandy, a graduate student at MIT. Shuwen Yue, an MIT postdoc, Changhwan Oh, Gianmarco Terrones, and Chenru Duan, a Ph.D. student, are additional authors. D. Yongchul G., 22; and 22. Chung, an assistant professor of chemical and biomolecular engineering at Pusan National University.
MOF modeling.
Because of their porous structure, MOFs are attractive to scientists because they can be used for a variety of gas-related tasks, including gas storage, gas separation, and gas conversion. Recently, researchers have started to look into using them to deliver drugs or imaging agents inside the body.
The organic molecules known as linkers, which join the secondary building units together, and the secondary building units, which are organic molecules incorporating metal atoms like zinc or copper, are the two main components of MOFs. Similar to LEGO building blocks, these components can be assembled in a variety of ways, according to Kulik.
“There is a combinatorial explosion of different possible metal organic framework materials because there are so many different types of LEGO blocks and ways to assemble them,” she claims. By carefully choosing how to assemble various parts, you can actually regulate the overall structure of the metal organic framework.”.
Trial-and-error design is currently the most popular method for creating MOFs. For the purpose of designing these materials, researchers have more recently started to experiment with computational methods. The majority of these studies have relied on forecasts of how well the material will perform in a specific application, but they frequently neglect to consider the material’s stability.
As Kulik points out, “A really good MOF material for catalysis or for gas storage would have a very open structure, but once you have this open structure, it may be really hard to make sure that material is also stable under long-term use.”
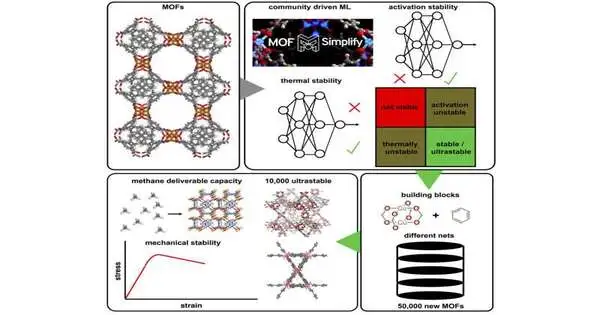
In a 2021 study, Kulik described a new model she had developed by scouring a few thousand papers on MOFs for information on the temperature at which a specific MOF would degrade and whether a given MOF could withstand the conditions required to remove the solvents used to synthesize it. Based on the structure of the molecules, she trained the computer model to predict those two characteristics, known as thermal stability and activation stability.
Through the use of this model, Kulik and her students were able to locate 500 MOFs with exceptionally high stability for the new study. Then, they disassembled those MOFs into their most prevalent building blocks—120 secondary building units and 16 linkers.
The researchers created about 50,000 new MOF structures by recombining these building blocks using about 750 different types of architectures, many of which are not typically present in such models.
According to Kulik, “one of the things that was unique about our set was that we looked at a lot more diverse crystal symmetries than had ever been looked at before, [and] we did so using these building blocks that had only come from experimentally synthesized highly stable MOFs.”.
Ultrastability.
The researchers then predicted the stability of each of these 50,000 structures using computational models, and they found 10,000 of them to be extremely stable in terms of both thermal stability and activation stability.
The “deliverable capacity,” a metric for a material’s capacity to store and release gases, was another factor they considered when screening the structures. Because methane capture could be useful for removing it from the atmosphere or turning it into methanol, the researchers used methane gas for this analysis. They discovered that the 10,000 ultrastable materials they had discovered had good methane deliverable capacities and were also mechanically stable, as determined by their anticipated elastic modulus.
According to Nandy, “Designing a MOF necessitates taking into account a variety of stability types, but our models allow for a nearly cost-free prediction of thermal and activation stability.”. We offer a new method to find promising materials by also comprehending the mechanical stability of these materials.”.
The researchers also found a few building blocks that frequently result in more stable materials. A molecule containing the rare-earth metal gadolinium was one of the secondary building blocks with the greatest stability. Another was a porphyrin, a sizable organic molecule with four interconnected rings that contains cobalt.
Currently, Kulik’s lab is working with students to synthesize some of these MOF structures and test them for stability, potential catalytic ability, and gas separation capability in the lab. Researchers who wish to test the materials for use in their own scientific applications can do so by utilizing the database of ultrastable materials that the researchers have made available.
More information: Heather J. Kulik, A Database of Ultrastable MOFs Reassembled from Stable Fragments with Machine Learning Models, Matter (2023). DOI: 10.1016/j.matt.2023.03.009. www.cell.com/matter/fulltext/S2590-2385(23)00111-X