NIST researchers have decided to find new materials that can draw planet-warming carbon dioxide (CO2) out of the air, a method known as “direct air catch,” with the ultimate goal of lessening the dangers of environmental change.
Direct air catch materials currently exist, yet they either cost a lot of cash or consume a lot of energy to be sent on a worldwide scale. NIST researchers are utilizing virtual experiences to quickly screen speculative materials that have never been blended yet but could have the perfect actual properties to make this innovation adaptable.
“The customary approach to screening materials is to blend them, then test them in the lab, yet that is painfully slow,” said NIST compound designer Vincent Shen. “Virtual experiences accelerate the disclosure cycle hugely.”
Shen and his partners are likewise developing new computational techniques that will speed up the hunt much more.
“Our objective is to create more efficient modeling approaches that extract as much information as possible from simulations. We believe that by sharing these tools, we will speed up the computational discovery process for other scholars working in this subject.”
NIST chemical engineer Vincent Shen
“We want to encourage more effective displaying strategies that concentrate as much data out of a game as possible,” Shen said.”By sharing those strategies, we desire to accelerate the computational revelation process for all analysts who work in this field.”
Direct air catch is significant on the grounds that mankind has currently significantly changed Earth’s air — 33% of all the CO2 in the air arrived because of human action. “Carbon capture is a method for switching a portion of those outflows and assisting the economy with becoming carbon unbiased more rapidly,” said NIST scientist Pamela Chu, who drives the organization’s as of late sent off carbon catch drive.

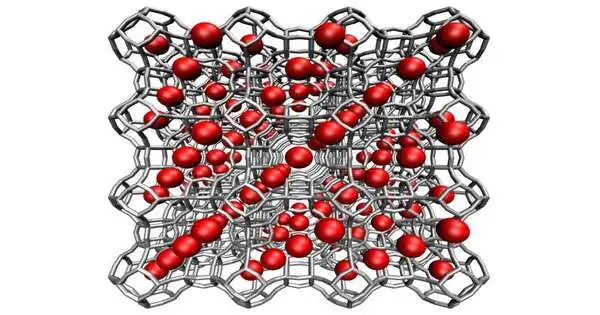
A delivery from a virtual experience of a permeable glasslike material called Zeolitic Imidazolate Framework-8, or ZIF-8. Credit: NIST
Whenever CO2 is captured, it tends to be utilized to make plastics and carbon strands or joined with hydrogen to create engineered fuel. This purpose requires energy, yet can be carbon neutral whenever fueled by renewables. Where sustainable power isn’t accessible, the CO2 can be infused into profound land developments determined to keep it trapped underground.
NIST researchers use virtual experiences that compute a potential catch material’s liking for CO2 compared with different gases in the air. That permits them to foresee how well the catch material will perform. The recreations likewise create pictures that show how carbon capture deals with a sub-atomic scale.
Permeable glasslike materials show a specific commitment to catching CO2. These materials are comprised of iotas organized in a rehashing three-layered design that leaves voids between them. In this reasonable outline, the dim bars address a glasslike material, and the red circles are the voids.
Electrons are conveyed unevenly inside the gem structure, creating an electric field that is alluring in certain spots and awful in others. The shapes of that field are determined by the types of iotas in the gem and their mathematical arrangement.Assuming every one of the powers lines up perfectly, CO2 atoms will be brought into the voids of the gem by electrostatic fascination.
Permeable glasslike materials can be blended with different sorts of iotas, and the molecules can be arranged into various calculations. The stages are basically unending. Virtual experiences permit researchers to investigate that huge universe of potential outcomes.
“We can envision materials that have never existed and foresee how they would perform,” said NIST compound designer Daniel Siderius.
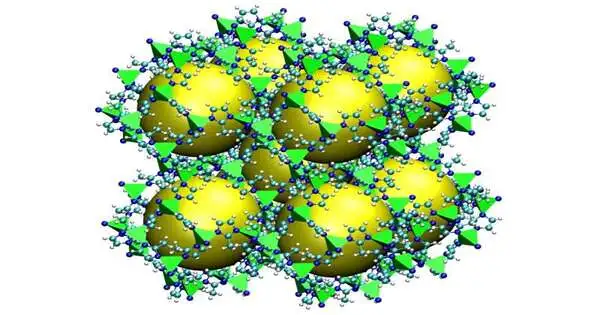
A delivery of the ZIF-8 material with voids addressed as yellow circles. Credit: NIST
The virtual experiences combine physical science standards with factual strategies to predict which path CO2 particles will take when they come into contact with a catch material—whether they will be drawn into the voids, diffuse out into the surrounding air, or simply bob around haphazardly in a state of harmony.
Most recreation techniques predict how a framework will behave at a given temperature, strain, and thickness.Yet, displaying techniques from NIST permit analysts to extrapolate that information to various circumstances.
“Let’s assume you’ve assessed the way of behaving at one temperature, yet you need to realize what might occur at an alternate temperature. “Normally, you would need to run another recreation,” Siderius said. “With our devices, you can extrapolate to various temperatures without running another recreation. That can save a ton of time and effort.
As of now, the best-performing process for modern-scale carbon capture works by foaming air through a compound arrangement. Yet, catching the CO2 is just a portion of the cycle. It then must be taken out of the arrangement, so it tends to be put away, so the arrangement can be utilized once more. This requires warming the answer to a high temperature, which takes a ton of energy.
NIST scientists desire to find a material that will remove CO2 from the air at typical temperatures and tensions yet discharge it because of relatively small changes in intensity or strain. The ideal cycle will be minimal expense, both monetarily and energy-wise, and will not produce harmful final results.
“We haven’t hit on the best materials yet,” Siderius expressed, discussing the more extensive local area of researchers who are figuring this issue out. Yet, there are a ton of likely materials out there, and new recreation strategies can assist us with finding them more rapidly.
Provided by National Institute of Standards and Technology