Tetraphenylammonium, with every one of the four hydrogens of ammonium (NH4+) supplanted with benzene rings, has neither been found in nature nor artificially orchestrated, raising doubt about whether it could exist. Here, we prevailed with regard to combining tetraphenylammonium interestingly, exhibiting its steady presence. The manufactured methodology utilized in this review, revolutionary coupling, might be appropriate for the blend of different related ammoniums with high underlying curiosity. The review was published in Nature Communications.
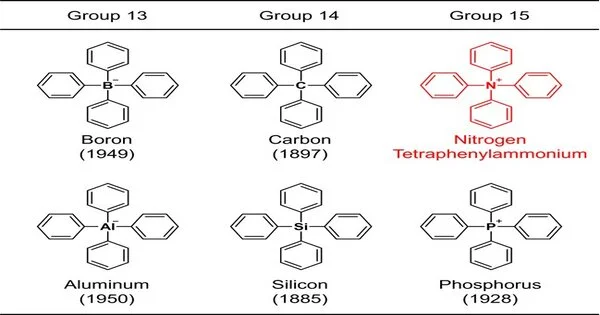
Because the benzene ring is a constituent of natural mixtures, a construction consisting solely of a common component in addition to the benzene ring is regarded as the most basic synthetic skeleton. Because of their significance, compound blends of such particles have been examined since the beginning of natural science. For instance, the design in which four benzene rings are clung to a delegate component (boron, carbon, aluminum, silicon, or phosphorus) of gatherings 13 to 15 in the occasional table (Fig. 1) was blended over a long time ago, and the most established engineered report goes back 137 years.
These skeletons are on the whole alluded to as “tetraphenyl,” and that implies that the design contains 4 benzene rings. At the point when the focal component is nitrogen, ammonium, NH4+, is viewed as the parent particle. Such a compound is called tetraphenylammonium. This compound, truth be told, a particle has an exceptionally straightforward synthetic design that even a novice in natural science can undoubtedly envision.
By the by, it has ended up being undeniably challenging to falsely make this design, and no manufactured reports with clear construction recognizable proof have been distributed. Moreover, since it has not been found in nature, it has not been clear as of recently whether tetraphenylammonium can exist by any means. Distributions have given the impression that accepts its presence for a moment and notices only its utilization without depicting its blend or securing strategy. Compound data sets contain just the substance structure. As a result, this article is occasionally alluded to as if it were known at the time. In any case, as a general rule, nobody has really noticed it, making tetraphenylammonium a “ghost particle.”
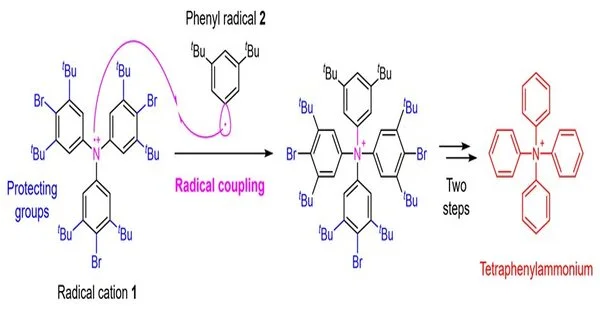
Figure 2. Tetraphenylammonium union procedure utilizing revolutionary coupling. Kanazawa University is to blame.
In this review, an exploration group at the Faculty of Pharmaceutical Sciences, Kanazawa University, has empowered the combination of tetraphenylammonium by laying out an original engineered system. The basic point in the amalgamation of tetraphenylammonium is the expansion of the fourth phenyl group to the nitrogen iota that as of now has three phenyl bunches appended. It was believed to be challenging to accomplish this union with customary strategies. In the current review, thusly, the examination group applied a method called revolutionary coupling and utilized a technique of responding to the extreme cation 1 arranged from a triphenylamine subordinate with the phenyl revolutionary 2 (Fig. 2).
Subsequently, albeit the yield was basically as low as 0.1%, the examination group prevailed with regards to playing out the ideal substance transformation. In such extreme couplings, exceptionally responsive revolutionaries structure bonds with one another, which enjoys the benefit of empowering bond arrangements that couldn’t be accomplished by other strategies. Then again, it has the drawback that it is hard to control selectivity in light of the fact that the reactivity is excessively high, prompting different side responses.
Hence, in this combination, to smother as much as could be expected the side response of bond arrangement on the carbon of revolutionary cation 1, the exploration group likewise formulated the presentation of safeguarding gatherings) that cause steric obstruction. At long last, a sum of five stages of compound change of a known triphenylamine subsidiary, the beginning material for the union, was brought out through the presentation of the safeguarding gatherings, revolutionary coupling, and resulting evacuation of the safeguarding gatherings, prompting tetraphenylammonium.
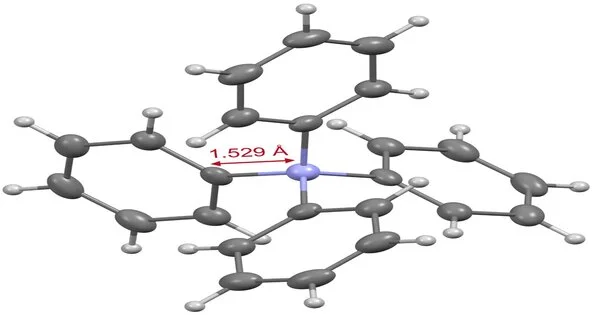
Figure 3. X-beam gem design of tetraphenylammonium. Kanazawa University is to blame.
In light of the information obtained from different instrumental examinations, the construction of tetraphenylammonium was affirmed. X-beam crystallography) uncovered that the bond length between the nitrogen iota and the phenyl bunch carbon particle contained in this article is just 1.529 (Fig. 3).
Since this bond length is more limited than that of a tetraphenyl structure containing another component (boron, carbon, aluminum, silicon, or phosphorus), it is obvious that the nitrogen iota of tetraphenylammonium is in a more spatially restricted environment than different components. This three-layered deterrent is viewed as one of the elements that make it hard to build this skeleton. Furthermore, our findings revealed that tetraphenylammonium has a high tolerance to extremely acidic and critical conditions.
The current review has demonstrated that tetraphenylammonium does, as a matter of fact, exist and can be synthetically combined. If the huge scope amalgamation of this article and its subsidiaries is acknowledged from now on, it might actually be applied in different exploration fields as a natural cation with high compound strength. Moreover, the extreme coupling system utilized in this study might be relevant to the amalgamation of other related ammoniums that couldn’t be made up to this point.