Antitumor agents must eradicate cancer cells while safeguarding healthy tissue and causing no harmful side effects. These requirements could be met by a novel strategy based on “self-immolative” polyferrocenes, which are copolymers that separate into their components as soon as they enter a tumor cell. According to a report published in the Angewandte Chemie International Edition journal by a research team, the drugs they contain then synergistically cause an abrupt increase in free radicals and incapacitate the defenses of tumor cells.
A group led by Xianglong Hu and Shiyong Liu at the College of Science and Innovation of China (Hefei, China) integrated two synergistically helpful particles together into a copolymer. At one end, the copolymer chains are hydrophilic, while at the other, they are hydrophobic.
They aggregate into nanoparticles in aqueous environments, with the hydrophilic outer portions protected by polyethylene glycol moieties and the hydrophobic inner ends pointed inward. Non-toxic polyethylene glycol is frequently used in pharmaceuticals and cosmetics. The nanoparticles are prevented from being rapidly broken down by immune system components in the blood by the polyethylene glycol layer.
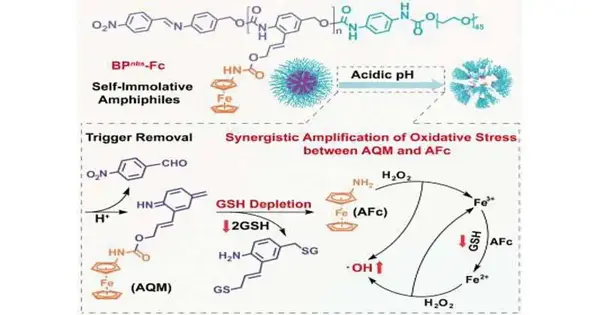
The nanoparticles remain intact and inactive in healthy tissues. “Self-immolation” is only initiated by tumor cells’ significantly more acidic environment. The particles go to pieces, and the hydrophobic finishes of the polymer chains split into their singular structure blocks. Azaquinone methide (AQM) units with aminoferrocene sidechains make these. A unique iron complex known as a sandwich complex is aminoferrocene: As the “slices of bread,” the iron atom is the “filling” between two aromatic, flat, five-membered carbon rings.
Inside the cancer cells, glutathione is thus initiated. Glutathione is an antioxidant and a trap for free radicals that aids in the removal of foreign substances from cells. Glutathione attacks, binds, and separates the iron sandwiches from the azaquinone methide units. Hydroxyl radicals (OH) are produced when this complex reacts with the cells’ hydrogen peroxide (H2O2). In this interaction, the divalent iron in the complex is oxidized to trivalent iron, which the glutathione lessens back to FeII—aa lethal cycle that consumes the glutathione and unexpectedly delivers a high centralization of hydroxyl extremists in the cell.
The tumor cells undergo severe oxidative stress as a result of the synergy between these two processes, which damages and kills them. Tests performed by the group both in vitro and in mice with cancer showed that this effectively hindered growth and development with immaterial aftereffects. Chemodynamic therapy (CDT) for tumors may open up new avenues with this strategy.
More information: Jie Xu et al, Self‐Immolative Amphiphilic Poly(ferrocenes) for Synergistic Amplification of Oxidative Stress in Tumor Therapy, Angewandte Chemie International Edition (2023). DOI: 10.1002/anie.202303829