Reciprocal light electron microscopy (CLEM) is an integral asset in bioimaging, as it consolidates the capacity to picture living cells over huge fields of view with sub-atomic explicitness by utilizing light microscopy (LM) with the high spatial goal and ultrastructural data of electron microscopy (EM). Researchers must ensure that biomolecules of interest are labeled with probes that are visible in both LM (typically by fluorescence) and EM (using electron-dense material) in order to accurately highlight and position them in CLEM. However, there are a number of problems with the probes that are currently available, such as their lack of integrity and stability under LM (photobleaching).
A group of researchers, led by Professor Paola Borri from Cardiff University and Professor Paul Verkade from the University of Bristol in the United Kingdom, have developed a brand-new CLEM method that does not require the use of fluorescent probes and instead makes use of small gold nanoparticles as a single probe that is visible in both LM and EM and has high contrast and photostability.
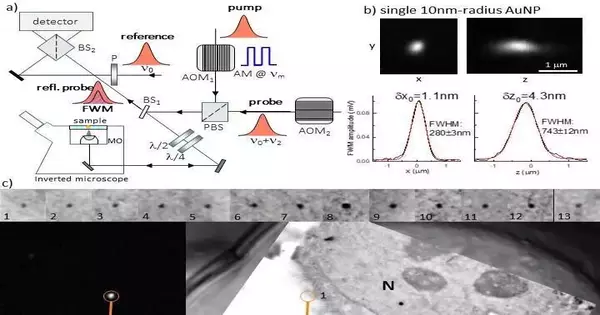
They used an optical microscope made by Professor Borri’s group for LM. Four-wave mixing (FWM) is the nonlinear optical response that gold nanoparticles exhibit under this microscope. The researchers were able to locate individual small gold nanoparticles as single probes of the epidermal growth factor protein in human cancer cells using this strategy. These gold nanoparticles were located with nanometric precision and were free of background using light microscopy. They were also correlatively mapped to the associated transmission electron microscopy image with high accuracy.
FWM-CLEM permits highly accurate correlative microscopy workflows without the use of unstable fluorophores or additional fiducial markers thanks to the background-free and photostable FWM response of individual gold nanoparticles, which is well visible in electron microscopy due to their electron dense composition. This greatly simplifies sample preparation protocols.
The researchers sum up the functional guidelines of their FWM magnifying lens as follows: “IIn its general structure, FWM is a third-request non-straight light-matter connection peculiarity wherein three light fields cooperate in a medium to produce a fourth wave. In this case, we employ a method in which the optical absorption peak of a gold nanoparticle is in resonance with all light waves having the same center frequency. We make use of a pump and probe—short optical pulses of about 150 fs each—generated by the same laser source for both excitation and detection. The identified FWM can be perceived as a siphon-prompted change in the capacity of the gold nanoparticle to send and dissipate light, which appears as an adjustment of the test bar dispersed by the molecule within the sight of the siphon.
“FWM detection is extremely high contrast and photo-stable. At imaging speeds and excitation powers compatible with live cell imaging, we are able to detect individual small gold nanoparticles within scattering and autofluorescing cells and tissues with a sensitivity that is only limited by photon shot noise. This method can detect gold nanoparticles with a radius of up to 5 nm. We have also discovered that FWM is a sensitive reporter of the shapes of gold nanoparticles, which makes it possible for shape recognition multiplexing in future applications.”
“FWM-CLEM opens new exciting possibilities for correlative light electron microscopy workflows, overcoming existing limitations with fluorescent probes and/or fiducial markers that are not required in our method,” the researchers conclude. For the most part, we accept that FWM-CLEM will have a far-reaching influence. FWM, for instance, opens the exciting possibility of tracking single viruses over extended observation times, background-free, deep within living cells and tissues, and then using CLEM to pinpoint events of interest (such as genome release) in the context of the cellular ultrastructure.” This is possible when combined with existing methods to label virions with gold nanoparticles or even encapsulate them inside virions.
More information: Iestyn Pope et al, Correlative light-electron microscopy using small gold nanoparticles as single probes, Light: Science & Applications (2023). DOI: 10.1038/s41377-023-01115-4