Russian specialists have made sense of why researchers concentrating on raw petroleum and divine science—as well as sure rational matters—regularly run over certain particles integrating carbon, nitrogen, and hydrogen, yet not different mixes of these three components. The revelation changes what used to be a mix of erratic guidelines of natural science into a slick, independent, coherent framework in view of the ongoing major comprehension of quantum physical science.
Distributed in The Diary of Actual Science Letters, the review isn’t simply certain to satisfy numerous fussbudgets out there, yet it will truly direct astrophysicists toward new “synthetic species” in space—indeed, that is truly the very thing they’re called.
“In the event that you contemplate how natural science is frequently educated, it’s a piece like attempting to remember the business catalog,” said Skoltech Teacher Artem R. Oganov, the essential examiner of the review. “A few particles are steady and hence normal; others are not really. Some respond promptly, others do not. In any case, why? There are decisions to this kind of work; however, it’s anything but a flawless framework worked starting from the earliest stage—more like a complicated assortment of perceptions.”
“When you think about how organic chemistry is generally taught, it’s a bit like trying to memorize the yellow pages. Some compounds are stable and so common, while others are not. Some people respond quickly, while others do not. But why? There are rules to this type of labor, but it’s not a clean system created from the ground up—rather, it’s a haphazard collection of observations.”
Skoltech Professor Artem R. Oganov, the principal investigator of the study.
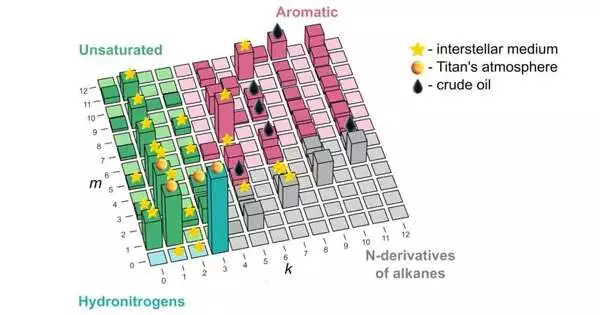
“Our review cures what is happening and tells the best way to make sense of and anticipate these things from the first standards for the arrangement of carbon, nitrogen, and hydrogen,” he went on. “Presently, there’s one little diagram that makes sense of anything we’ve seen so far in space or in unrefined petroleum, to say the least, all things considered.” The hidden standard was acquired from atomic material science and nanoscience and distinguishes “sorcery” particles—those that have lower energy than the particles of the closest structures.
The laws of material science say that matter will, in general, accept the most reduced energy state accessible. That implies the pretty, profoundly coordinated frameworks, which somebody has invested a ton of time into, will generally corrupt when just let be (sorry, guardians). Food is a genuine model: sooner rather than later, a succulent apple loaded with delectable energy loses it either to the climate by rotting or, ideally, to somebody who can see the value in it.
Deprived of its energy, the mixtures in the apple change into the recognizable response items, with the most perceptible particle among them—think smell—being skatole, named after the Old Greek word for crap. In this way, in fact, what makes a particle horrible is exactly how low the energy of the atom that preceded it was ready to fall over the response.
Skatole (C9NH9) is near being a “wizardry” particle (and one more atom with similar creation and a marginally unique game plan of iotas ended up being sorcery). Unexpectedly, the rotten skatole is utilized in frozen yogurt and aroma, yet in extremely small amounts—think about what for? For its fragrance.
While the lead creator of the paper, Skoltech expert’s understudy Elizaveta Vaneeva from Oganov’s Material Disclosure Lab, was hesitant to dig into the entire skatole part of the review, she liberally remarked on where the new compound strength guide could really be utilized in science and industry. “The expectations of our model concur well with the rundown of atoms tracked down in unrefined petroleum and space.
“In any case, while oil has been completely researched, with regards to the interstellar medium and planetary environments, we could possibly give astrochemists a few clues about what to search for, and it’s a lot quicker and more straightforward to find new particles when you have a rundown of up-and-comers within reach.”
“Concerning the innovative applications, one could envision natural physicists attempting to incorporate an economically helpful compound that has a place in the class we were checking out,” Vaneeva added. “This could be a natural color, for instance, a blue shade. And on second thought, rather than drearily doing examinations to sort out which compound would be steady, they could utilize our strategy, which depends on crucial quantum substance computations, to foresee the probable applicants. The strategy has no analogs, is very quick, and presently its expectations have been tried against the distributed astro- and petrochemistry information.”
Later on, the specialists plan to extend their approach to dealing with other natural frameworks, like amino acids—the structure blocks of DNA and RNA—and proteins.
More information: Elizaveta E. Vaneeva et al, Prediction and Rationalization of Abundant C–N–H Molecules in Different Environments, The Journal of Physical Chemistry Letters (2023). DOI: 10.1021/acs.jpclett.3c01753