In drug development and screening, the capability of isolating and controlling the biodynamics of individual cells is crucial. However, in order to accurately predict drug-single cell interactions and performance, pre-existing experimental reports in single-cell drug screening must still include multiple-dose gradient studies. Shaofei Shen and a group of Chinese researchers in medicine and life sciences developed a multi-concentration gradient generator to solve this issue, which they describe in a new paper published in Microsystems and Nanoengineering.
In order to comprehend the single-cell effects of single or combined doses of 5-fluorouracil and cisplatin anticancer drugs on human hepatoma and breast cancer cells, they utilized a gradient generator and a single-cell capture array. In order to efficiently screen single-agent chemotherapy agents and construct combinatorial therapy regimens, the instrument provided a simple and dependable platform for studying the appropriate dosage of various drug candidates at the single cell level.
Developing a brand-new platform for drug screening
Drug screening methods provide a visible solution for treating and preventing infections in humans. Expansive examination endeavors have shown that microfluidic chip innovation offers a microanalysis stage for simple access and biocompatibility. The majority of microfluidic systems facilitate dose-dependent cellular responses at various drug concentrations and provide a powerful instrument for studying cell populations at the level of individual cells. A single-cell microfluidic drug screening platform for multiple drug doses was created by Shen and colleagues by optimizing a multistage microfluidic device and a single-cell capture array.
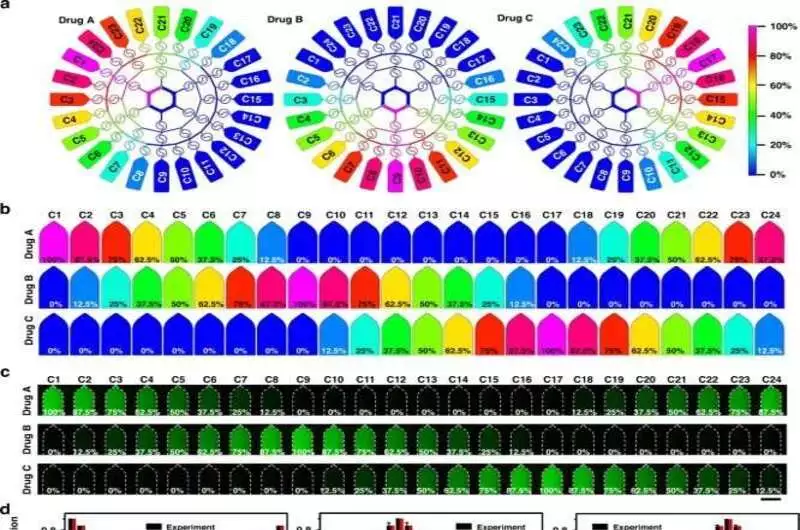
Under various flow clow conditions, the generation of a microfluidic drug concentration gradient a) Simulation imaging of the device’s drug concentration gradient generation pr ess When the flow rate was 10 L/mdrugsdru B, andre poured into three inlets. b) A numerical simulation of the formation of a drug concentration gradient in 24 microchambers c) Fluorescence experiments’ 24-microchamber concentration gradients Using luciferin as a model drug, fluorescence was used to characterize the system’s substance transport, distribution, and concentration gradient. Scale bar: 1200 m.onditions, the generation of a microfluidic drug concentration gradient a) Simulation imaging of the device’s drug concentration gradient generation process. When the flow rate was 10 L/min, drug A, drug B, and drug C were poured into three inlets. b) A numerical simulation of the formation of a drug concentration gradient in 24 microchambers c) Fluorescence experiments’ 24-microchamber concentration gradients Using luciferin as a model drug, fluorescence was used to characterize the system’s substance transport, distribution, and concentration gradient. Scale bar: 1200 μm. (d) A computer-simulated fluorescence concentration value in 24 microchambers The ten parallel experiments’ standard deviations are indicated by the error bars. e) A fluorescence experiment and numerical simulation of the drug concentration in the first nine microchambers under various flow conditions reveal a linear relationship. Credit: Microsystems & Nanoengineering (2023). DOI: 10.1038/s41378-023-00516-0.
In this work, the theoretical concentration gradient that was generated was calculated and verified through a fluorescent experiment. They utilized cisplatin and 5-fluorouracil, two chemotherapeutic specialists, as model medications for single or multidrug combinatorial chemotherapy on human bosom cell carcinoma cells and human hepatoma cells at the single-cell level. For studying pharmacological functions and single-cell research, the system offered an adaptable and tightly controlled instrument.
Designing the microfluidic platform
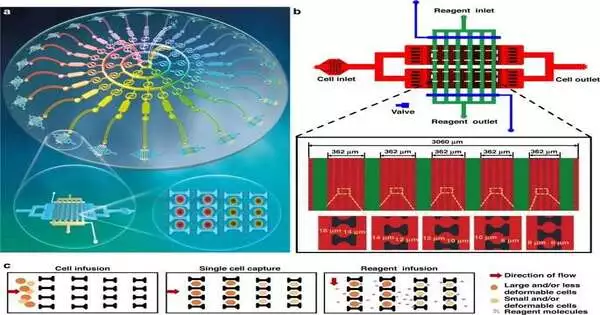
Shen and the group developed and constructed the microfluidic platform, which contained 24 single-cell capture devices. They used the device to successfully generate a series of drug concentration gradients. This allowed them to examine the optimal dosage for both a single drug and multiple drug combinations while simultaneously screening two drugs using the concentration gradient generator.
According to a previous study by the same team, the bioengineers helped the single-cell capture device contain a two-dimensional array with cell and reagent portals for reagent import and suspension.
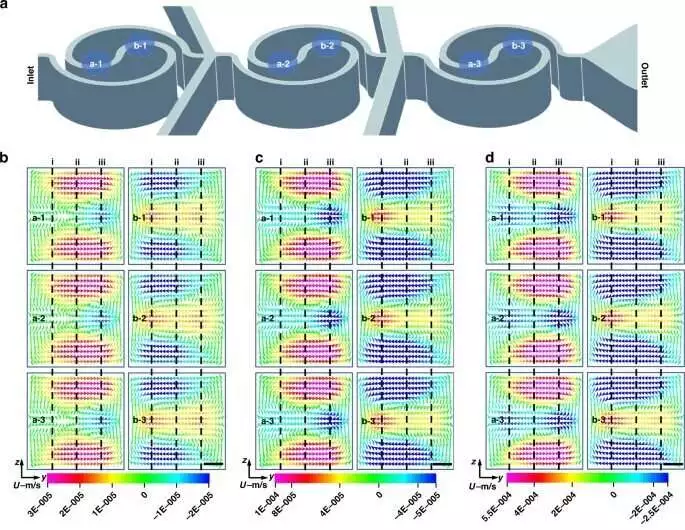
Dean flow is causecaused by a spiral mixer and Tai Chi. a) Draw a sketch of the channels of the spiral mixer. b-d) Senior member stream recreation at various situations in the channel when the stream rate waL10 L/min (b),L0 L/min (c), and L L/min (e). Scale bar: 10 m.d by a spiral mixer and Tai Chi. a) Draw a sketch of the channels of the spiral mixer. b-d) Senior member stream recreation at various situations in the channel when the stream rate was 10 μL/min (b), 20 μL/min (c), and 50 μL/min (e). Scale bar: 10 μm. e Quantitative examination of Dean flow across sections The positions depicted by the dotted lines in Fig. are where the results are obtained. 3b–d. Credit: Nanoengineering and Microsystems (2023). DOI: 10.1038/s41378-023-00516-0
Three-concentration gradient formation
The researchers investigated drug distributions in 24 microcavities by evaluating the device’s capacity to create three concentration gradients through numerical simulations and fluorescein experiments. Utilizing a modified magnifying instrument, they accumulated fluorescent pictures and utilized imaging programming to comprehend the information assembled for each chamber.
The recreation showed the development of three groups of indistinguishable medication fixations in the planned gadget and their rate. Fluorescein experiments were used to confirm the concentration gradient distribution in 24 liquid storage chambers alongside the simulations. They infused two fluorescent specialists into the chip from the passages and saw how the trial results concurred with the aftereffects of mathematical reproductions.

Tumor cell responses to multiple-gradient dosages of two drugs in the largest filter units of single-cell capture structures (5-FU, DDP) a) Fluorescence images of HepG2 cells were obtained by AO/PI staining after two hours of continuous treatment with various drug concentrations. b) Fluorescence images of MCF-7 cells were obtained by acridine AO/PI staining after two hours of continuous treatment with various drug concentrations. 800 μm. Credit: Microsystems & Nanoengineering (2023). DOI: 10.1038/s41378-023-00516-0.
Tai Chi-spiral mixer–induced Dean flow in microfluidics
The group utilized a Kendo winding blender in the fixation slope generator and a consolidated Dignitary stream in the bending channels for microfluidic uses of liquid guideline as a progressive way to deal with blend, purge, and spotlight responses proficiently and cost-effectively.
They constructed a multiple-concentration platform for single-cell-level drug screening to observe the combined and distinct effects of the anticancer drugs cisplatin and 5-fluorouracil. This allowed them to obtain a stable and effective multidrug concentration gradient for single-cell drug screening.
Screening drugs for interactions with single cells
The bioengineers used a drug-containing medium with different concentrations of the two types of cells: human bosom cell carcinoma cells and human hepatoma cells, in single-cell catch microfluidic gadgets and in customary Petri dishes. Utilizing methods of double fluorescence staining with acridine orange and propidium iodide, they confirmed the viability of the cells and observed variations in the activity of the cells when they were exposed to various drug concentrations to demonstrate how the survival rate of the tumor cells improved as the drug concentration decreased in the culture devices.
For effective investigations of their susceptibility to drugs, tumor cells were unaffected by cell interactions at the single-cell level, whereas the cell vitality of healthy cells was negatively correlated with the drug dose.
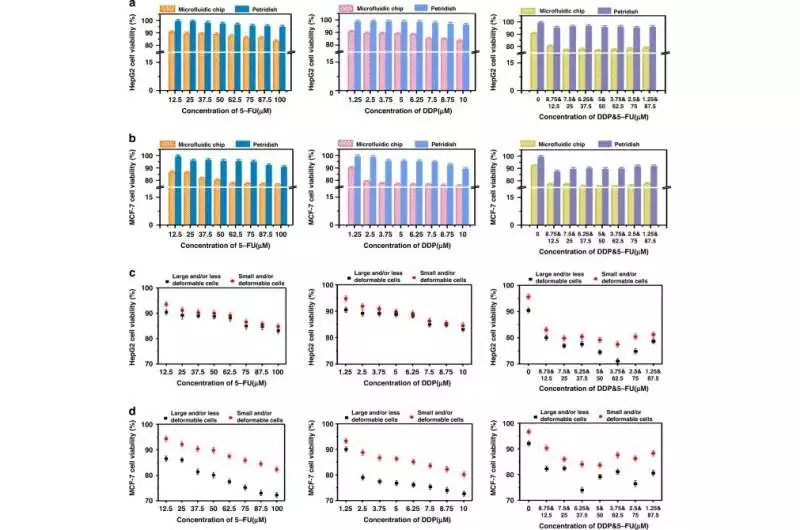
Quantitative comparison of the cell viability of two drugs at various dosage gradients (5-FU, DDP) a, b; quantitative examination of MCF-7 cell reasonability (a) and HepG2 cell feasibility (b) in single-cell level culture in microarrays and Petri dishes (c) Quantitative comparison of MCF-7 and HepG2 cell viability in the various biomechanical heterogeneities of tumor cells at the individual cell level. Credit: Microsystems & Nanoengineering (2023). DOI: 10.1038/s41378-023-00516-0.
The team observed a stronger synergistic effect of two drugs when compared to monotherapy alone on the cells in the platform. Microfluidic single-cell capture devices that offer effective monotherapy and combination therapy strategies also contain this phenomenon. For instance, the significance of drug screening to investigate the heterogeneity of tumor cells across various drug gradients is demonstrated by the fact that cells exposed to 5-fluorouracil underwent dose lethality to inhibit DNA synthesis and cell mitosis. The work altogether affects more extensive natural and preclinical investigations, including malignant growth, immature microorganism partition, and medication revelation.
Outlook
As a result, Shaofei Shen and colleagues developed a single-cell-level multifunctional microfluidic drug screening device that was both simple and effective. A drug generator with a concentration gradient and a single-cell capture array served multiple functions in the device.
They combined the platform’s single-cell capture device to administer two distinct doses of anticancer drugs to two distinct cancer cell lines. In order to develop efficient chemotherapeutic strategies, the work provides a solid foundation from which to investigate the sensitivity of various anticancer agents at the level of single cells and stem cells.
More information: Shaofei Shen et al, Construction of multiple concentration gradients for single-cell level drug screening, Microsystems & Nanoengineering (2023). DOI: 10.1038/s41378-023-00516-0
Leonard I. Zon et al, In vivo drug discovery in the zebrafish, Nature Reviews Drug Discovery (2005). DOI: 10.1038/nrd1606