Green hydrogen can be created through water electrolysis innovation, which utilizes environmentally friendly power to part water into hydrogen and oxygen without transmitting carbon dioxide. Nonetheless, the creation cost of green hydrogen is right now around $5 per kilogram, which is a few times higher than dim hydrogen obtained from petroleum gas.
For the functional utilization of green hydrogen, advancements in water electrolysis innovation are expected for the acknowledgment of the hydrogen economy, particularly in Korea, where the usage of environmentally friendly power is restricted for geological reasons.
Dr. Kyung Joong Yoon’s examination group at the Energy Materials Exploration Focal Point of the Korea Foundation of Science and Innovation (KIST) has fostered a nanocatalyst for high-temperature water electrolysis that can hold a high flow thickness of more than 1A/cm2 for a significant stretch of time at temperatures above 600°. The work is distributed in the Substance Designing Diary.
“Our newly developed nanomaterials achieved both high performance and stability for high-temperature water electrolysis technology, and it can contribute to lowering the production cost of green hydrogen, making it economically competitive with gray hydrogen in the future.”
Dr. Kyungjoong Yoon of KIST.
While the debasement systems of nanomaterials at high temperatures have been subtle up to this point, the group recognized the key reasons for the unusual way of behaving nanomateirals and effectively settled issues, at last further developing execution and dependability in practical water electrolysis cells.
The electrolysis innovation can be ordered into low- and high-temperature electrolysis. While low-temperature electrolysis working at temperatures below 100°C has for quite some time been created and is innovatively more fully grown, high-temperature electrolysis working above 600°C offers higher productivity and is considered a cutting-edge innovation with a solid potential for additional expense reduction.
Be that as it may, its commercialization has been obstructed by the absence of warm strength and the deficient lifetime inferable from high-temperature debasement, like erosion and primary twisting. Specifically, nanocatalysts, which are broadly used to work on the presentation of low-temperature water electrolyzers, immediately weaken at high working temperatures, making it hard to actually involve them in high-temperature water electrolysis.
To overcome this restriction, the group developed a new nanocatalyst-engineered strategy that stifles the development of unsafe mixtures, causing high-temperature corruption.
By methodically breaking down the nanoscale peculiarities utilizing transmission electron microscopy, the specialists distinguished explicit substances causing extreme primary changes, for example, strontium carbonate and cobalt oxide, and effectively eliminated them to achieve exceptionally stable nanocatalysts with regards to synthetic and actual properties.
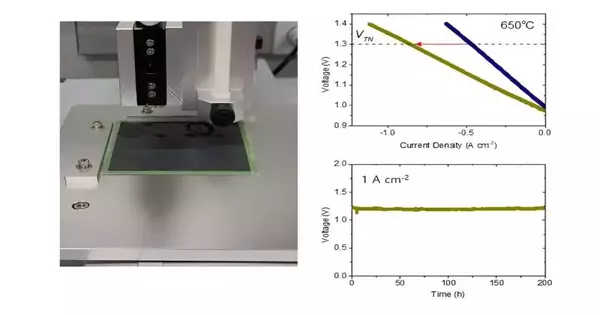
At the point when the group applied the nanocatalyst to a high-temperature water electrolysis cell, it dramatically increased the hydrogen creation rate and worked for over 400 hours at 650° without debasement. This method was likewise effectively applied to a commonsense huge region water electrolysis cell, affirming serious areas of strength for its increase and business use.
“Our recently evolved nanomaterials accomplished both elite execution and security for high-temperature water electrolysis innovation, and it can add to bring down the creation cost of green hydrogen, making it financially aggressive with dim hydrogen later on,” said Dr. Kyungjoong Yoon of KIST.
“For commercialization, we intend to foster computerized handling procedures for large-scale manufacturing in collaboration with industry cell makers.”
More information: Mi Young Park et al, In situ synthesis of extremely small, thermally stable perovskite nanocatalysts for high-temperature electrochemical energy devices, Chemical Engineering Journal (2023). DOI: 10.1016/j.cej.2023.146924