A long time ago, we saw a forward leap in current science.
An American researcher found that control of the Cas9 protein brought about a quality innovation deserving of a science fiction film: CRISPR.
Consider it some atomic scissors fit for cutting and altering the DNA of people, creatures, plants, microbes, and infections.
The potential is immense and covers anything from erasing innate illnesses to creating crops ready to endure environmental change.
Nonetheless, similar to some other new innovations, CRISPR has had its difficulties. One of the primary moves has been to make the innovation as viable as possible and to ensure the scissors just slice where we need them to.
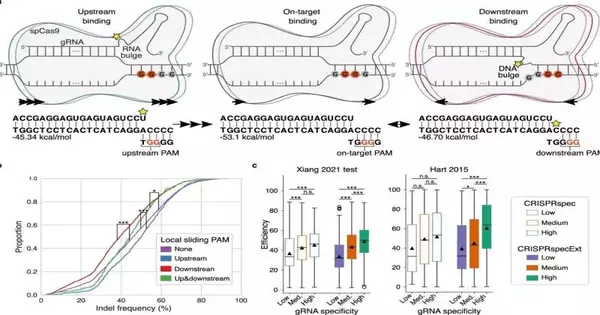
We have depicted new systems behind CRISPR.
Two new examinations from the College of Copenhagen, led by analysts from Aarhus College, can assist with tackling these issues.
“We have depicted new systems behind CRISPR,” explains Teacher of Bioinformatics Jan Gorodkin from the Branch of Veterinary and Animal Sciences.
“We are currently ready to make sense of why a few off-targets, which are accidental cuts somewhere else in the genome, are more viable than on-targets, which is the cut at the planned spot. We’ve also discovered what different DNA clusters around the track can mean for how well the Cas9 protein cuts the DNA.Ideally, this information will prepare us for a more viable and more secure utilization of CRISPR. “
“We already know that when the link between the guide RNA and the DNA is too weak, CRISPR does not work. We now know that too strong a relationship is also problematic.”
Jan Gorodkin
So how does CRISPR function? First, a researcher will prepare a piece of engineered RNA called the aide RNA. This is then joined to the Cas9 protein, which will carry out the errand of cutting the DNA. The aide RNA scouts for the matching DNA area. When the aide RNA has found the ideal place, Cas9 will cut the DNA string. Presently, the researcher can embed any engineered piece of DNA into the emptied spot.
In the event that Cas9 and the aide RNA hit the objective, the researchers allude to it as being on track; assuming they hit somewhere else, they are askew.
Today, CRISPR is, with regards to medication, mostly used to concentrate on how qualities and medications work in the lab and is as yet not broadly utilized in human treatment. Nonetheless, in the long haul, the thought is to involve CRISPR in the treatment of specific hereditary illnesses.
The mystery of viable off-targets has been solved.
In one of the two new examinations, the analysts tried to decide the most ideal way for the aide RNA to join to the DNA, getting it done as viable as could be expected — since, supposing that the cut isn’t adequately powerful, the researchers can not alter the DNA.
“We definitely realize that CRISPR doesn’t actually work when the connection between the aid RNA and the DNA is excessively frail. “Presently, we have discovered that too solid a bond is risky as well,” says Gorodkin. “In the two cases, the quality scissors are excessively frail and incapable.”
The scientists then recognized a span where the connection between the aid RNA and the DNA is neither too solid nor excessively frail, yet spot on, thus bringing about scissors with amazing sharpness.
Strangely, this perception can likewise be utilized to make sense of why a few off-targets show more grounded CRISPR action than their planned on-targets—that is, the reason the scissors are sharper for a few off-focuses than for the on-targets,” Gorodkin makes sense of.
“This is on the grounds that too many solid on-targets don’t fall inside the right restricting energy span. Yet, in the event that you eliminate a portion of the energy from these solid bonds, which is what’s going on at the off-target locales, you can get into the span right, bringing about a more impressive impact and hence a shaper scissor at the off-targets. “
The review has likewise recognized the ideal position of the Cas9 protein for accomplishing the best cut.
Before Cas9 can cut the DNA, the protein should bind to a particular piece of the DNA string. DNA is comprised of four unique nucleotides: A, C, G, and T, and Cas9 can tie to a succession with two sequential G nucleotides.
Presently, the scientists affect Cas9 of various successive G nucleotides — a circumstance where it is difficult to raise a ruckus around town in light of the fact that each two continuous G’s seek restricting with Cas9.
At the point when there are a few G’s ‘upstream’, that is, before the succession that Cas9 was planned to tie to, the cut will be more viable. Yet, when there are a few G’s ‘downstream’, that is, after the planned succession for Cas9 to tie to, the cut is less effective, “makes sense to Postdoc Giulia Corsi.”
Corsi believes that learning more about how CRISPR works will make it easier to locate Cas9 later on.This should also help to limit the number of possible side effects.
“We might want to have the option to foresee the cut, further develop target altering and kill off-targets, which muddle the improvement of new medications by requiring loads of assets and can bring about aftereffects that happen when you cut some unacceptable quality,” says Corsi.
Off-targets can be unsafe — and they are under-explored.
The subsequent review centers around off-targets. Here, the scientists fostered a strategy for gauging the effectivity of off-targets.
To quality-control a CRISPR experiment, researchers will normally choose fewer anticipated off-focuses for testing. Utilizing the new innovation, in any case, they will actually want to test a much larger number of off-targets, and this is supposed to accelerate the improvement of new medications with fewer secondary effects.
Utilizing the new strategy, the analysts tried 8,000 likely off-focus for 110 CRISPR guide RNAs during the time spent being converted into human medication. They observed that around 10% of the 8,000 potential off-targets were, truth be told, off-targets.
“We found undeniably more off-focus than we would have had the option to utilize existing strategies,” Gorodkin makes sense of.
Besides, 37 of these off-targets are situated in disease-related qualities, which increases the gamble that creating medications will become more difficult or even unthinkable. Moreover, accidental cuts in these qualities might try and prompt disease as a potential secondary effect.
“Analysts should have the option to recognize such off-targets and select other aide RNAs which don’t have these or some other basic asymmetry,” says Gorodkin.
Incredible requirement for more examination of off-targets
As per Gorodkin, this shows that there is an incredible requirement for more examination of off-targets.
“I would contend — and some might differ — that off-targets are very under-explored. “My impression is that current examinations on quality altering frequently miss the mark on complete devices and examinations expected to show that there are no askew impacts in their examinations,” he says.
As per Jan Gorodkin, the new strategy will have an extraordinary effect in the future by more readily looking at reads up for off-targets.
In the last 10 years, we have made a major stride towards having the option to alter the genome. Presently, we are currently improving our techniques to be more secure and more viable. The last option likewise upholds the green change, as genome adjustments, for example, of cells utilized underway, can prompt more savvy use of assets. “
The two examinations are published in Nature Correspondences.
More information: Giulia I. Corsi et al, CRISPR/Cas9 gRNA activity depends on free energy changes and on the target PAM context, Nature Communications (2022). DOI: 10.1038/s41467-022-30515-0
Xiaoguang Pan et al, Massively targeted evaluation of therapeutic CRISPR off-targets in cells, Nature Communications (2022). DOI: 10.1038/s41467-022-31543-6
Journal information: Nature Communications