According to Weill Cornell Medicine researchers, inhibiting a critical signaling route in brain-resident immune cells may reduce brain inflammation and hence halt the disease process in Alzheimer’s and other neurodegenerative disorders. The findings suggest the possibility of new therapeutic techniques for neurodegenerative illnesses, which are highly frequent in older persons but have no effective, disease-modifying treatments to far.
Brain inflammation, particularly the activation of immune cells termed microglia in the brain, has long been recognized as a prevalent characteristic of neurodegenerative disorders. Another common hallmark of these illnesses is the growth of aberrant, thread-like clumps – “tangles” – of a neuronal protein called tau.
The researchers demonstrated that tau tangles aid in the inflammatory activation of microglia via a multifunctional signaling route known as the NF-κB pathway in their study, which was published in Nature Communications. Inhibiting microglial NF-κB signaling in a tau-based Alzheimer’s mice model significantly reduced inflammatory immune cells and corrected the animals’ learning and memory impairments.
“Our findings suggest restraining overactive NF-κB may be a good therapeutic strategy in Alzheimer’s and other tau-mediated neurodegenerative diseases,” said senior author Dr. Li Gan, director of the Helen and Robert Appel Alzheimer’s Disease Research Institute and the Burton P. and the Judith B. Resnick Distinguished Professor in Neurodegenerative Diseases in the Feil Family Brain and Mind Research Institute at Weill Cornell Medicine.
Our findings suggest restraining overactive NF-κB may be a good therapeutic strategy in Alzheimer’s and other tau-mediated neurodegenerative diseases.
Dr. Li Gan
Tau tangles are discovered within neurons in Alzheimer’s, Parkinson’s, Pick disease, progressive supranuclear palsy, frontotemporal dementia, and other neurodegenerative illnesses. Experiments have revealed that tangles can seed the production of additional tangles when injected into animal brains, causing a chain reaction in which the tangles spread to other brain regions. Autopsy investigations in Alzheimer’s and other “tauopathies” show that the spread of tangles closely matches disease progression.
The specific significance of tangles in brain cell damage has never been fully understood. However, previous research has revealed that tau tangles can interact with microglia in a way that causes the microglia to become inflammatory and disease-associated. Microglia, which normally try to devour tau tangles, become relatively inefficient in this inflamed state. Much of the tau is not digested, but rather excreted by microglia in forms that tend to create new tangles.
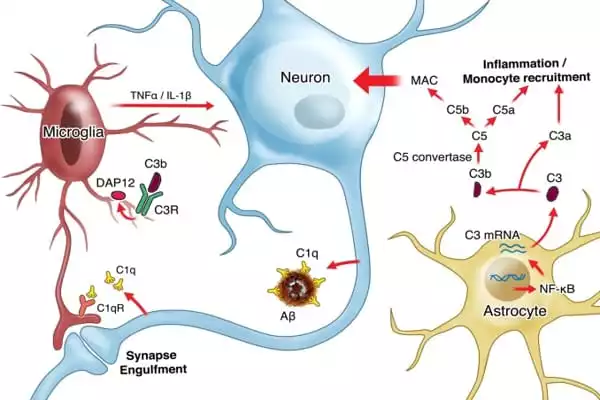
In the new study, Dr. Gan and her team found evidence from cell culture and mouse experiments that tau tangles push microglia into this disease-linked inflammatory state mainly by activating the NF-κB signaling pathway within them. In a Alzheimer’s mouse model with tau-tangle mainly driven by seeded tau, they showed that keeping the NF-κB pathway overactive in microglia enhanced the seeding and spread of tangles, which propel further NF-κB activation. By contrast, shutting off NF-κB blocked this vicious cycle, and markedly lessened the spread of the tangles.
In another tau mouse model, with tau tangle formed in aged neurons, the researchers showed that the inactivation of microglial NF-κB shifted the microglia almost entirely out of their inflammatory, disease-associated state, restoring a much more normal cell appearance and pattern of gene activity. This shift, which suppresses microglia from disgorging toxic tau seeds, strikingly, prevented key cognitive/memory deficits the mice normally develop in this model.
“Taken together, our experiments suggest that tau’s toxic effects on cognition require microglial NF-κB signaling,” said co-senior author Dr. Wenjie Luo, associate professor of research in neuroscience in the Appel Alzheimer’s Disease Research Institute and the Feil Family Brain and Mind Research Institute at Weill Cornell.
Many experimental Alzheimer’s treatments have tried to slow or stop the disease process by targeting amyloid plaques and, more recently, tau tangles over the last two decades. All of these initiatives have so far failed in large-scale clinical studies. According to Dr. Gan, the current data suggest that future treatments regulating hyperactive microglial NF-κB signaling may fare better.
Her lab is now conducting additional research to better understand how microglial NF-κB signaling, which influences the activity of at least hundreds of other microglial genes, damages neurons and leads to cognitive and memory problems. The researchers will look into methods to limit certain components of hyperactive NFκ-B signaling without interfering with the regular function of the brain’s immune cells.