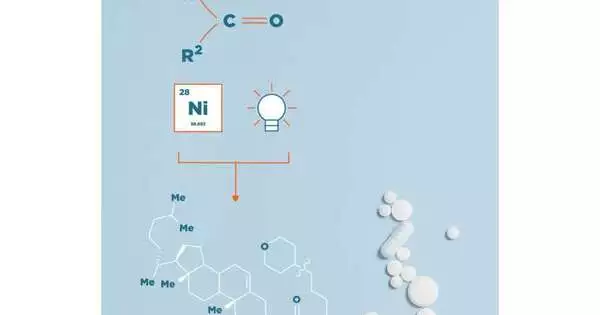
Specialists have distributed a paper depicting, interestingly, the utilization of ketones as alkyl cross-coupling synthons.
For Xin-Yang Lv and Dr. Roman Abrams, analysts in Prof. Martin’s gathering at the Institute for Chemical Research of Catalonia (ICIQ), staying aware of logical writing is a wellspring of motivation for the improvement of new manufactured procedures. Making a new distribution one step further, the researchers in Prof. Martn’s gathering have conceived another system that works with the making of natural particles of pharmacological interest. The technique has been published in the open-access journal Nature Communications.
The system created by the Martin bunch makes ketones accessible to physicists to be utilized as alkyl cross-coupling synthons. “This strategy gives a huge adaptability to scientists and drug specialists in the combination of natural particles, by moving past the old-style reactivity of ketones and towards the utilization of this plentiful class of compound as cross-coupling accomplices,” makes sense to Prof. Ruben Martin, head of an examination group at ICIQ and ICREA teacher.
“By moving beyond the classical reactivity of ketones and toward the utilization of this abundant class of substance as cross-coupling partners, this approach affords considerable flexibility to chemists and pharmacists in the synthesis of organic compounds.”
Prof. Ruben Martin, leader of a research group at ICIQ and ICREA professor.
The creativity of the work lies in the utilization of ketone determined dihydroquinazolines as extremist forerunners. The specialists have utilized a double synergist approach, utilizing nickel and a photoredox impetus to produce and trap extremist intermediates for the resulting cross-coupling, framing another C bond in a specific and controlled way.
Having the option to involve generally bountiful and effectively preparable ketones as cross-coupling handles, along with the gentle response conditions, is vital to the expected far-reaching utilization of this new procedure. The creators give instances of the utilization of this technique in the context of other manufactured handles in the paper.
“The technique stands apart for its incredible breadth and wide application for the amalgamation of natural particles. “It is another methodology for the development of designs of incredible interest for restorative and drug science,” says Prof. Martin.