Methane, as the primary part of shale gas, petroleum gas and burnable ice, is among the most encouraging energy assets for creating high-esteem synthetics. In any case, it is as yet testing to enact methane under gentle circumstances because of the great balance and low polarizability of methane atoms.
As of late, an exploration group driven by Prof. Deng Dehui and Assoc. Prof. Yu Liang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has accomplished profoundly effective room-temperature methane change to fluid C1 oxygenates over ultrathin two-layered (2D) Ru nanosheets with grid-bound Cu iotas.
This study was published in Chem Catalysis on August 24.
Ultrathin 2D metallic nanosheets are promising grid materials for making dynamic places for methane actuation by keeping heteroatoms in the cross section. In any case, the barely controllable fitting of the coordination climate for the bound heteroatoms in the 2D nanosheets makes it difficult for the development of viable dynamic locales for methane actuation.
“This discovery presents an approach for developing effective catalysts for the activation of C-H bonds in light alkanes by creating edge-confined active centers in metallic nanosheets,”
Prof. Deng Dehui
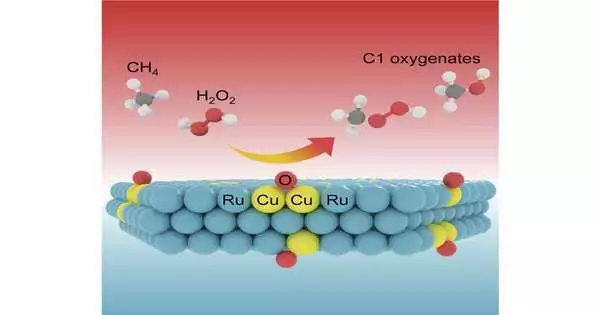
In this review, the scientists fostered the impetuses by binding Cu iotas in ultrathin 2D metallic Ru nanosheets through a novel system of honorable metal-prompted decrease components, which empowered a profoundly specific methane change to fluid C1 oxygenates under room temperature.
They achieved the formation of fluid C1 oxygenates (CH3OOH and CH3OH) over the Ru11Cu impetus to a limit of 1533 mmol g-1Cu(surf.) h-1 with more than almost 100% selectivity using H2O2 as the oxidant by precisely changing the substance of the bound Cu iotas to improve their coordination climate.
Various spectroscopic investigations and first-principles estimations revealed that bi-facilitated site oxygen species produced on the Ru edge-bound Cu locales could simply separate the C-H obligation of methane with a moderately low energy obstruction, and thus power the methane transformation at room temperature via a free extreme system.
“This study gives a system to plan effective impetuses by building edge-bound dynamic places in metallic nanosheets for the enactment of C-H bonds in light alkanes,” said Prof. Deng.
More information: Jinchang Fan et al, Boosting room-temperature conversion of methane via confining Cu atoms in ultrathin Ru nanosheets, Chem Catalysis (2022). DOI: 10.1016/j.checat.2022.07.025