The substance is present on two-thirds of the Earth’s surface, but billions of people lack access to clean, drinkable water.
An electrified version of dialysis is used in a new purification system developed by Beckman Institute for Advanced Science and Technology researchers to separate salt and other undesirable particles from the potable product. The method, which has been successfully applied to wastewater and is planned for expansion into rivers and seas, consumes 90% less energy and saves money than its competitors. The research was presented in ACS Energy Letters.
If, by some stroke of good luck, taking salt from water was pretty much as straightforward as waving a monster magnet over the Pacific or filtering fluid through a super-fine sifter, When the tricky mineral disintegrates, the partition cycle—ddedicated desalination in logical circles—tturns out to be more costly and utilizes more energy.
“We need a low-energy, low-cost method of purifying drinking water that is useful to the communities that need it the most.” “I see our solution as a platform for addressing both the energy and water crises,”
Xiao Su, a Beckman researcher and an assistant professor of chemical
The removal of impurities and organic matter—the tiny specks suspended in a scoop of unfiltered ocean water—increases energy and cost budgets, making desalination even more difficult.
“We require a low-energy, low-cost, and useful method of purifying drinking water for the most in-need communities. Xiao Su, a Beckman researcher and an assistant professor of chemical and biomolecular engineering at the University of Illinois Urbana-Champaign, stated, “I see our solution as a platform to tackle both the energy and water crises.”
When de-salting water, undesirable elements like sodium, chloride, organic matter, and a variety of atomic stowaways must be removed through filtration or evaporation. A straightforward kitchen experiment demonstrates that boiling salted water results in the liquid evaporating and the salt remaining as a solid, briny crust. Heat, on the other hand, successfully performs this trick.
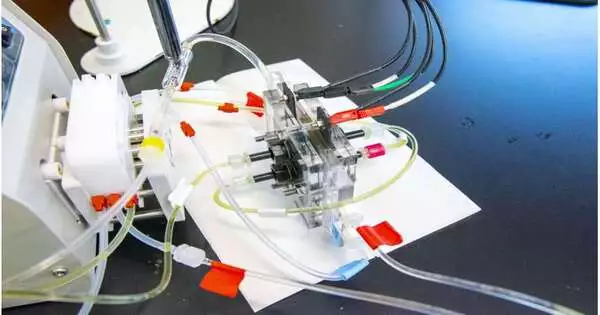
Su and his coworkers used a different strategy: electrodialysis. Electrodialysis removes salts and organic matter from wastewater to produce a clean, drinkable product, much like blood dialysis, which flushes salt and other toxins from our veins in a manner similar to that of the kidney.
Electrodialysis is a useful technique for desalination, but it frequently requires a lot of energy. This is largely attributable to its most prominent reaction, water splitting, which splits water molecules into two parts: a hydroxide with a negative charge and a proton with a positive charge. Like a moth to an oppositely charged flame or a scrap of metal to a magnet, splitting the water forces the mineral’s movement in a specific direction because the building blocks of salt have their own charges.
Rather than a magnet, however, electrodialysis utilizes charged particle trade films, so named in light of the fact that main particles (iotas with a positive or negative electric charge) can go through. Ion-exchange membranes are one of the most expensive components of electrodialysis because they need to be maintained meticulously and replaced frequently.
Su and his partners tried to sanitize water without the energy cost of electrodialysis or the monetary cost of particle trade films. As a result, they made two major changes to the conventional approach.
To save energy, the specialists smoothed out the salt partition process with a substance peculiarity called a redox response. Redox is a portmanteau of the words reduction and oxidation, which in chemistry mean adding electrons to make a negative charge and subtracting electrons to make a positive charge, respectively. Before wastewater is filtered and purified, it looks like a special polymer-based material is added to start a redox reaction.
The outcomes are revolutionary chemically. Rather than parting water atoms into decidedly and adversely charged cuts to cajole out the salt, the redox response changes the charge of the whole water particle all at once, accomplishing a similar level of pungent partition with around 90% less energy than conventional water-parting.
The researchers switched from conventional ion-exchange membranes to nanofiltration membranes, which are stronger and less expensive, to increase energy efficiency while also saving money.
Tests at a provincial water treatment plant showed that the scientists’ strategy can effectively filter wastewater. Expanding into saltwater and brackish water sources like rivers and groundwater is planned for the future.
Redox-inspired electrodialysis is designed to work well with solar panels because it requires little energy. Su stated that applications in climate-affected regions “where low-cost, low-energy desalination is very much needed” can benefit from its favorable performance in hot climates.
“Water scarcity is a global issue that will not go away overnight. However, “we are taking a step toward a solution that is attainable and scalable,” he stated.
Up until this point, the scientists have tried their technique on examples of different liters. However, they long to expand into a larger pond.
Su stated, “We have the appropriate conditions, the appropriate membrane, and the appropriate polymer.” The next step is to clear the way for these devices to be used in real-world water treatment because the science is there. I accept everything looks good for that, and I’m eager to witness it.”
More information: Nayeong Kim et al, Redox-Copolymers for Nanofiltration-Enabled Electrodialysis, ACS Energy Letters (2023). DOI: 10.1021/acsenergylett.3c00482